I-131 Ablation for Thyroid Neoplasms
General Principles
I-131 has a physical half-life of 8.05 days. It decays by high energy gamma photon (364 keV) and particulate emissions (beta particle). The beta emission has an average energy of 192 keV (max energy = 607 keV) and the beta particle will deposit the majority of its energy within 2.2 mm of its site of origin. Because it is primarily concentrated in thyroid tissue, I-131 can be used in the treatment of thyroid cancer. In order to ablate the thyroid bed in post surgical patients, between 30,000 to 100,000 rads is needed to be delivered to the remaining thyroid tissue. Two important determinants of the success of thyroid ablation are the mass of remaining thyroid tissue in the neck, and the initial dose rate to this tissue. Dose rates below 300 rad/hr and/or more than 5 gm of residual thyroid tissue are associated with a lower success rate for complete ablation [2]. A dose of at least 7000 rads is desired at sites of residual disease. Nodal metastases require a dose of at least 8500 rads, with little effect shown when the delivered dose is below 3500 rads.
Although it is generally recommended to limit treatment to yearly intervals, if necessary, therapy for mets may be repeated as necessary every 3 to 6 months for up to 5 to 10 treatments.
Indications
Near-total thyroidectomy spares the posterior capsule on the side contralateral to the carcinoma in an attempt to preserve parathyroid tissue. Ablation therapy with I-131 is performed for the following indications:
* To destroy the small amount of thyroid tissue remaining in the neck after surgery
* For the treatment of functional metastases
* For the treatment of patients with elevated thyroglobulin levels, but a negative I-131 scan [3,33].
To make an intelligent decision regarding treatment options, attention must be paid to prognostic factors. In very low risk patients: Age under 45 years, primary lesion less than 1.5 cm, no evidence of vascular, lymphatic, or capsular invasion, and a well-differentiated tumor- radioiodine ablation of the thyroid bed may not provide additional benefit. TSH suppression and follow-up may be sufficient in this subgroup of patients. [4] Waxman feels that, regardless of the size of the lesion, it is useful to ablate all patients with pure follicular neoplasms, as these are frequently more aggressive.
Contraindications to I-131 therapy
- Pregnancy: Radioiodine freely crosses the placenta. The fetal thyroid extracts/concentrates iodine after the 12th week and the radiation will destroy the thyroid gland and result in severe hypothyroidism. Additionally, activity in the maternal bladder causes significant fetal irradiation.
- Breast feeding: Both iodine and pertechnetate are excreted in breast milk
Technique
In patients being considered for ablation therapy, a pre-treatment diagnostic I-131 scan is performed to assess for the presence of metastatic lesions. It is important to note that I-131 given for this diagnostic scan can exert a negative effect on the uptake of the therapeutic dose by residual thyroid bed tissue and functioning metastases- this is referred to as "Thyroid Stunning". In fact, the higher the diagnostic dose used, the greater the possible subsequent decrease in uptake of the therapeutic dose. Most people feel that only 2 to 3 mCi of I-131 (and certainly no more than 5 mCi) should be used for the pre-ablation diagnostic scan. Some authors suggest only 1 mCi should be used for the diagnostic scan, because even a 3 mCi dose can exert a negative effect on ablation therapy [5]. Unfortunately, lower diagnostic doses can miss more metastatic lesions which would be detected with larger doses (10 mCi). [6,7,8]. To decrease the effect of stunning, it may be beneficial to lengthen the period of time between the diagnostic scan and I-131 therapy to approximately one week [3]. However, other individuals feel that stunning is generally not observed until 8 to 10 days following the diagnostic scan and that the therapeutic dose should be given immediately following the diagnostic study [Dr. O'Mara at SNM meeting 2001]. Although stunning is presumed to lessen the therapeutic effect of I-131 ablation therapy and be associated with a lower success rate for remnant ablation [28,29], it does not appear to have been reported to be associated with a decreased patient survival.
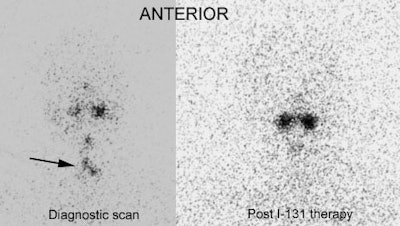
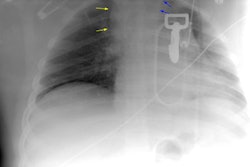
Thyroid Stunning: Post thyroidectomy diagnostic I-131 scan revealed neck bed activity (oral-pharyngeal activity can also be seen). Following treatment with 100 mCi of I-131 the post-therapy scan demonstrated no evidence of tracer uptake in the neck indicative of thyroid stunning. |
|
Diagnostic quality pre-ablation scans can also be performed using I-123. A dose of 1.5 to 2 mCi is used and whole body and dedicated anterior and posterior neck/chest images are obtained at 24 hours. By using I-123 the radiation dose to the thyroid gland is substantially decreased (by approximately 100 times) and I-123 has not been reported to cause thyroid stunning. Unfortunately, I123 is considerably more expensive than I131 and its use may not be reimbursed.
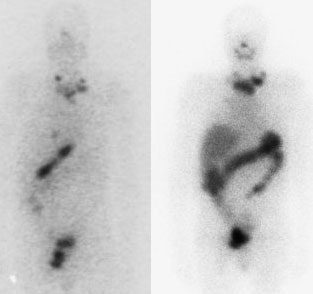
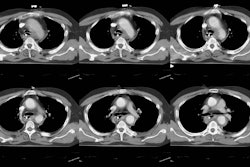
I-123 Diagnostic scan: The diagnostic scan on the left was performed using I-123. Extensive pathologic nodal uptake is seen within the lower neck and mediastinum. A separate focus of increased tracer accumulation is seen over the right upper abdomen (not seen on post-therapy scans- possibly due to superimposed liver activity). The post-I-131 therapy scan (right) demonstrates uptake in the nodal metastases and diffuse hepatic tracer activity due to metabolism of radiothyroxine. |
|
When scanning these patients, Dr. Waxman feels that 20 minute spot images of the head, neck, chest, abdomen, and pelvis are more desirable than total body scans with a moving camera. Dr. Mike McBiles (Brooke Army Medical Center) feels that whole body scans with a table speed of 5 cm/ min. are comparable to spot images, but this varies with detector size, given the same imaging distace from the detector. Dr. McBiles recommends phantom studies should be performed to determine the optimal table speed for individual systems.
I-131 Treatment Protocols for Thyroid Carcinoma:
The activity of radioiodine used for ablation of thyroid remnants and treatment of metastatic disease is not standardized and several treatment options exist.
1- Aggressive/Restrained (Beierwaltes)
Fixed amounts of radioiodine are given based upon the location of metastases-
- Residual thyroid bed activity only: 100 mCi
- Regional Mets (Cervical Nodes): 150-175 mCi
- Lung Mets: 175-200 mCi
- Skeletal Mets: 200 mCi
2- High Dose:
Individually tailored to keep the blood dose just below 200 rads. Generally, patients receive about 300 mCi. This treatment is based on the assumption that mets may lose their ability to concentrate iodine, so the largest and safest dose possible should be administered at the first therapy.
3- Dosimetry
Dosimetry is utilized to determine what activity the therapeutic dose should be based upon the individual patients radioiodine pharmacokinetics. The therapeutic dose is adjusted to compensate for patient to patient variability in the rate of iodine clearance. Conditions such as renal failure, ascites, or pleural effusions can all result in prolonged retention of I-131. Patients with bulky functional metastases may also retain I-131 longer than usual as they will produce large amounts of radiothyroxine [27]. Using dosimetry the expected radiation dose to the whole body, blood, and sites of functioning thyroid tissue (thyroid bed, mets) is calculated. The therapeutic dose is then determined in order to maximize its effectiveness and improve patient safety. Because a dose of 1-2 mCi of I-131 is usually adequate for dosimetry it can be performed in conjunction with the pre-therapy diagnostic examination.
4- Low Dose: ALARA
I-131 30 mCi (1110 MBq) given repetitively as necessary. Patients do not require hospitalization. Reduction in cost and patient inconvenience are factors which make this form of treatment attractive.
Guidelines for maximum dose administration
The guidelines regarding the maximum activity which can safely be administered are: [11]
1- Blood dose should be no more than 200 rads
This limit is set to reduce marrow toxicity. Frequently (90%) doses of this level are associated with mild, transient decreases in blood cell counts, but no instances of permanent suppression have been reported.
2- Retained whole body activity of no more than 120 mCi at 48 hours (or 80 mCi in patients with lung metastases to avoid potential pulmonary fibrosis)
Previously, following therapy, patients had to be hospitalized until the retained radioactivity was less than 30mCi (1110MBq) or the metered exposure rate from the patient was less than 5 mR/hr at one meter. Patients can now be treated on an outpatient basis providing that certain exposure limits are maintained for individuals that may have contact with the patient (limit of 0.5 rem (5 mSv) TEDE to an individual due to radiations from the released patient) [25]. Click here for a further discussion.
Measures which increase the dose delivered to a metastatic lesion
1- TSH manipulation
TSH should be greater than 30 uU/ml prior to therapy. This will stimulate iodine uptake in functioning mets. TSH stimulation with human TSH should be considered for patients with TSH values less than 30. If the TSH level is less than 30, functioning thyroid mets may not be identified on diagnostic scans, and may not accumulate sufficient I-131 during therapy. In order to ensure adequate elevation in TSH levels, T4 should be discontinued for at least 4 to 6 weeks prior to the scan/therapy (the half-life of T4 in the blood is about 1 week). T3 (Cytomel) should be discontinued for at least 10 to 14 days (the half-life of T3 in the blood is about 18-24 hours).
2- Low iodine diet
Daily dietary iodine intake is maintained below 50 ug/day for 7-10 days prior to therapy. Patients avoid seafood, salt, and dairy products. These measures will decrease the extracellular iodine pool and increase uptake of radioiodine by about 2.5 times.
3- Lithium carbonate
Lithium suppresses the release of thyroid hormone from thyroid tissue and has been found to prolong the biologic T1/2 of I-131 especially in tumors with biologic half-lives of less than 6 days, with little effect on whole body exposure. When given for 1 week prior to therapy, it may serve to increase the radiation dose delivered to functioning thyroid tissue.
Immediate Follow-up Imaging
Follow-up whole body imaging is performed 7 to 10 days post high dose treatment. Scans performed before this time may miss metastatic lesions. Post therapy scans can detect new lesions in up to 15% of patients.
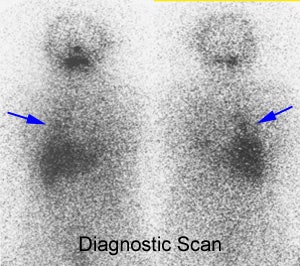
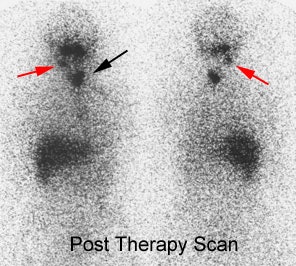
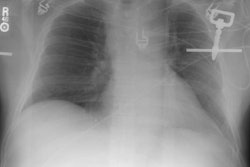
Post therapy scanning: The benefits of post-therapy scanning are demonstrated in the case below. The diagnostic exam (left) revealed abnormal tracer activity in the right lower lung (blue arrows) suggestive of metastatic disease. Hepatic activity is likely due to breakdown of radiothyroxine in the liver. The post-therapy scan (right) revealed numerous sites of abnormal tracer activity in the neck (red and black arrows) not evident on the diagnostic study. Note the lung activity is less apparent following therapy- possibly due to "stunning" from the diagnostic dose. |
|
Guidelines for Outpatient Thyroid Remnant Ablation:
In the past, patients were required to be hospitalized and in isolation after I-131 radioablation therapy until the dose rate from the patient was <5 mrems/hr, or the retained activity in the patient was <30 mCi. Patients can now be treated on an outpatient basis providing that certain exposure limits are maintained for individuals that may have contact with the patient (limit of 0.5 rem (5 mSv) TEDE to an individual due to radiations from the released patient). Click here for a further discussion.
I-131 Distribution
Normal sites of I-131 uptake:
Other than the thyroid bed, I-131 activity is also seen in the choroid plexus, nasal mucosa/nasopharynx (may be asymmetric), salivary glands, thymus, stomach, bowel, urinary bladder, and breasts. Ectopic thyroid tissue may also be identified. Diffuse liver uptake may be seen on post-therapy scans and is diagnostic of thyroxine production. (Thyroid hormone [T3,T4] is metabolized in the liver and activity can be seen there after 3 to 7 days). In post-ablation patients, liver activity may indicate that I-131 has been incorporated into thyroid hormone by functioning metastases. A large amount of residual thyroid activity in the neck, however, may produce similar findings. There is considerable uptake within lactating breasts. It is suggested that breast feeding be discontinued for several days prior to treatment in order to ensure mammary secretory activity has ceased in order to decrease the radiation dose to the breasts. Breast feeding should be discontinued following treatment [12]. Breast uptake has been described in up to 6% of non-lactating females and may be asymmetric. Breast uptake may be confused with lung metastasis [13].
False positive sites of I-131 uptake:
False-positive scans have been reported in association with uptake at sites of pathologic transudates, foci of inflammation, and uncommonly at sites of non-thyroid malignancy.
Benefits of I-131 Therapy
1- Decreases local recurrence
Local recurrence rates are decreased by about 50% in patients treated with greater than 90mCi of I-131, compared to those treated with surgery alone. The only exception to this is in patients who are at lower risk for local recurrence to begin with: i.e.: age under 45y, no distant mets, and primary tumor < 1.5cm [14]. Unfortunately, I-131 therapy has not been shown to statistically prolong survival.
2- Improves survival in patients following local recurrence
3- Prolongs survival in patients with lung or bone mets
However, the rate of actual cure is low [15]. Of note, bone metastases typically have little or no response to I-131 therapy [14], however, other authors have found that I-131 therapy can be used with curative intent in patients with bone metastases [31]. Patients with bone metastases that are most responsive to I-131 therapy tend to be younger (under 45 years of age) and have fewer bone lesions (less than 3 lesions) [31]. Overall, I-131 ablation has been reported to be less effective in patients younger than 40 years of age with metastases, however, these patients tend to live longer with metastatic disease anyway.
4- Eliminates the thyroid gland as source of thyroglobulin
I-131 ablation improves the sensitivity and specificity of post-therapy follow-up whole body I-131 scans and serum thyroglobulin assays. Thyroglobulin appears to be the most sensitive test for determining the presence of persistent or recurrent well differentiated thyroid cancer.The thyroid gland is the only source of thyroglobulin production in the body. Thyroglobulin levels should be undetectable in post ablation patients. A thyroglobulin level drawn while the patient is on thyroid hormone replacement may be falsely depressed and should preferably be measured after the patient has been withdrawn from replacement (during maximal TSH stimulation).
On follow-up, a rising thyroglobulin level implies the presence of functioning mets and will be elevated in 85-90% of patients with mets. Patients with bone metastases tend to have the high Tg levels, followed by those with pulmonary mets, and the least elevation is seen in patients with nodal mets. Serum thyroglobulin may rise to very high levels after surgical manipulation or needle biopsy, but will decline very quickly (with a half life of 22 hours). It is preferable to wait at least 1 week after these procedures prior to measuring Tg levels.
Thyroglobulin also rises after ablation or treatment of metastases with radioiodine and the levels tend to decrease more slowly (over months). Circulating antibodies to thyroglobulin can interfere with radioimmunoassays that utilize the second antibody method to measure thyroglobulin levels causing either falsely high or low results. Between 13 to 45% of patients with well differentiated thyroid cancer have anti-Tg antibodies which can decrease the sensitivity of serum Tg levels as an indicator for recurrent disease [16].
Acute Complications of I-131 Thyroid Ablation Therapy
The major and minor salivary glands concentrate iodine [26]. Sialoadenitis is characterized by pain, tenderness, and swelling of the salivary glands. It occurs in 10-33% of patients and is dose related [1]. The use of hard sour candies can reduce the incidence and severity of sialoadenitis. Following therapy, about of third of patients have subjective complaints of xerostomia during the first year, but up to 50% of patients will have objective evidence of reduced salivary function [26]. Objective reduced salivary gland function can persist beyond one year in 14% to 43% of patients [1,26], and objective evidence of xerostomia can be found in up to 14% of patients at 3 years post therapy [26]. Complete xerostomia occurs in only 4% [1]. The risk of salivary dysfunction increases with increasing accumulated dose- although the relationship is not linear [1,26].
2- Radiation parotiditis
3- Loss of taste (Acute/Chronic)
Between 27% to 50% of patients will suffer from a transient loss of taste or smell.
4- GI symptoms
Nausea and vomiting (seen in <1%)
5- Minimal bone marrow suppression
Transient pancytopenia is seen with a nadir at about 6 weeks post therapy. Recovery is spontaneous. Chromosomal abnormalities in peripheral lymphocytes are not uncommon (2-9%).
6- Radiation pneumonitis and pulmonary fibrosis
May rarely be a complication in patients with lung mets. The risk can be decreased by restricting the whole body retention at 48 hours to less than 80 mCi.
7- Radiation Gastritis/Cystitis
8- Thyroid storm
May occur with extensive follicular mets due to release of large amounts of thyroid hormone- typically seen 2 to 10 days post treatment
9- Transient amenorrhea
This occurs in older females and is not dose related. The typical dose to the ovaries is approximately 20 rads for a 100 mCi dose of I-131. Temporary ovarian failure has been described in about 25% of treated females, however, this may be related to other factors such as hypothyroidism and a perturbation of the pituitary-gonadal axis. I-131 therapy has not been shown to reduce fertility of treated patients or increase the risk of congenital abnormalities in their offspring [17].
10- Decreased testicular function/fertility
Decreased testicular function is seen in 10 to 50% of male patients receiving doses greater than 100 mCi. Spermatogonia are the most sensitive testicular cells to external radiation. The cumulative dose to the testes after a standard 100 mCi dose is between 50 to 150 rad. Good hydration and frequent voiding (every 2 hours) can minimize the testicular dose. Transient (over 6 to 12 months) increased serum FSH levels are found in one-third of male patients treated with I-131. FSH is a sensitive marker of germinal cell failure. Decreased spermatic motility was also observed.
Permanent elevations in FSH are seen to develop in patients receiving multiple treatments with high cumulative doses. Testicular atrophy with absent spermatogenesis has been reported in 3 patients that received doses between 450 and 820 mCi in association with external radiation. Two of these patients had large functioning pelvic metastases.
The bottom line is that I-131 therapy for thyroid carcinoma is associated with transient impairment of testicular germinal cell function. The damage may become permanent in patients that receive high cumulative radiation doses [18]
11- Cerebral edema or spinal cord compression
Seen in patients with CNS mets
12- Radiation sickness
Dose > 200mCi. Headache, nausea, vomiting.
13- Transient alopecia
Most likely related to disturbances in thyroid hormone status, rather than a radiation effect [1].
14- Chronic or recurrent conjuctivitis, keratoconjunctivitis, decreased lacrimal function
About one-quarter of patients treated will have subjective complaints of xerophthalmia
[1,26], and about 18% will have objective evidence of decreased lacrimal function after
one year [26]. Objective evidence for persistent keratoconjuctivitis sicca can be found in
up to 7.6% of patients three years following treatment [26]. Therefore, although decreased
lacrimal function is common after therapy, it is generally a transient side effect [26].
Chronic Complications of I-131 Thyroid Ablation Therapy
*There is NO increased risk of thyroid tumors and no evidence of reduced fertility or genetic abnormalities in patients offspring due to I-131 therapy. Genetic defects have been detected in 1.8% of patients with high-dose treatment, a percentage equal to that in the normal population [31].
1- Secondary Tumors
The risk of secondary tumor is low and lacks clinical impact [17,32]. The relative risk approximates 1.9 compared to the general population [31].
A- Leukemia
The risk for acute myelogenous leukemia is only minimally increased above the general population with a peak incidence 2 to 10 years post therapy (0.5% increased risk). Patients at risk are generally above the age of 50 and have received a dose of approximately 900 mCi. The risk is greatest when this large dose has been given over a short period of time (6 to 12 weeks). These patients have usually received a blood dose greater than 200 rads. It may also be possible that patients with thyroid cancer are at an increased risk for this type of malignancy regardless of the type of therapy they receive. To minimize the risk for leukemia there should be a 1 year interval between therapies and a total cumulative dose of administered activity should not to exceed 800 mCi [19] It is important to note that the mortality from recurrence exceeds that from leukemia by 4 to 40 fold.
B- Bladder carcinoma
There may be a slight increased incidence (6x) of bladder carcinoma if total dose was given over a short interval and exceeds 1000 mCi. The latency period is between 15 to 20 years.
C- Breast carcinoma
There may be a slight increase in breast carcinoma (3x) if the total dose was given over a short interval and exceeds 1000 mCi. This may be related to a genetic disposition for both breast and thyroid carcinoma, and not I-131 therapy.
D- Salivary carcinoma
There may be a very slightly increased risk for salivary tumors [17].
2- Hypoparathyroidism
Post I-131 treatment hypoparathyroidism is extremely rare.
Post-Therapy Hormonal Treatment
Thyroid hormone (T4) suppression therapy is effective in the management of differentiated thyroid carcinoma and doses sufficient to suppress TSH decrease the risk of recurrence. This is because well differentiate thyroid cancer responds to TSH stimulation, and grows more slowly in the absence of TSH.
Follow-up Post-Ablation Screening:
The overall risk of recurrence of thyroid cancer is about 20% [24]. The risk of recurrence is related to the age at diagnosis, extent of primary disease, size of the primary lesion, and the presence of metastases [24]. Surveillance for recurrence is a lifelong process. Following surgery and thyroid ablation with I-131, thyroid cancer patients are monitored for recurrence using serum thyroglobulin levels and whole body I-131 scanning. Thyroglobulin is a prohormone for thyroid hormone and it is produced exclusively by normal thyroid tissue and well differentiated thyroid cancers [33]. Thyroglobulin levels provide a means for detecting the presence of recurrent disease and for monitoring response to therapy. Compared with whole body I-131 scanning, thyroglobulin levels are a more sensitive test for detecting the presence of metastatic disease. In the absence of anti-thyroglobulin antibodies (and after the withdrawal of thyroid hormone), serum thyroglobulin levels have a sensitivity of about 80-90% for the detection of recurrent disease. Unfortunately, thyroglobulin levels provide no localization information and thyroglobulin levels may not be elevated in patients with only regional lymph node metastases [23]. In one study comparing I-131 scanning and thyroglobulin levels [20], thyroglobulin levels were elevated (above 10 ng/ml) in 88% of patients with metastatic lesions, while the I-131 scan demonstrated lesions in only 55% of the patients with proven metastases. There were no instances of a positive I-131 scan and a negative serum thyroglobulin level [20]. Other authors have demonstrated similar sensitivities for I-131 scanning. Tumor dedifferentiation plays a role in cases in which thyroglobulin levels are elevated, but the I-131 scan is negative. Obviously, the ability of thyroid cancer cells to produce thyroglobulin is maintained for a longer period of time in the process of dedifferentiation, than is the ability to take up iodine [23]. This can be explained by the fact that the mechanisms necessary for iodine storage (ie: iodination, iodization, and synthesis of thyroglobulin) are much more complex than is the synthesis of thryoglobulin alone [23]. Other factors such as the presence of micrometastases may also be responsible for negative I-131 scans.
In well differentiated neoplasms, thyroid hormone production requires thyroglobulin synthesis, a feature of follicular architecture. Iodide trapping without organification is much more common, however, and is found in the majority of papillary cancers. For these reasons, most metastatic deposits will concentrate iodine to some extent after total thyroidectomy. Diffuse hepatic uptake on post-ablation I-131 scans should be viewed as indirect evidence for the presence of metastatic disease, even if other foci of abnormal activity cannot be identified [23]. Hepatic activity is due to the breakdown of radioactive thyroid hormone by the liver [23].
Thyroglobulin levels in conjunction with the I-131 scan results also provide prognostic information. Patients with elevated thyroglobulin levels and a positive scan had the lowest overall mortality (44%), while those with metastases and a negative thyroglobulin level and negative scan had a mortality of 71%. Patients with elevated thyroglobulin levels and a negative scan had a mortality of 58%.
Some groups now advocate a trial of I-131 therapy in patients with elevated thyroglobulin levels, but negative I-131 scans [3,24,33]. In fact, current evidence suggests that post-ablation patients that are Tg positive and I-131 scan negative should be treated with 100-200 mCi of I-131. Therapy can result in a decrease in the thyroglobulin level (indicating that the treatment did have an effect on silent sites of metastatic disease) and post-therapy scans may demonstrate sites of metastases not evident on the pre-therapy scan in up to 59% of patients [21,33]. Whether this type of treatment can result in improved patient survival has not yet been demonstrated.
Other agents used for post ablation screening:
The detection of non-iodine avid disease in patients with thyroid cancer and elevated thyroglogulin levels poses a clinical and therapeutic dilemma. A neck ultasound examination can detect regional adenopathy and can be a useful first step in the evaluation of these patients. Several other imaging agents can also be used to assess for recurrent/metastatic disease in these patients.
Thallium-201 is one agent that has been used for imaging patients with thyroid cancer. Sensitivities for the detection of thyroid mets has ranged from 45 to 94%, with specificities between 94 and 97%. Thallium is probably best used to identify the presence of cervico-mediastinal lymph node mets. Thallium is less effective in identifying bone, liver, and diffuse pulmonary mets. One of the advantages of thallium imaging is that the patient does not need to discontinue hormonal replacement therapy. The exam may also be useful in patients with elevated thyroglobulin levels, but negative I-131 scans. Due to the high background activity in the abdomen and pelvis thallium imaging in these regions is seldom useful. It is probably best to limit thallium scans to the head, neck, and chest in order to obtain higher count density images of these regions. [22]
Tc-Sestamibi and Tc-Tetrofosmin have also been used for thyroid cancer detection
FDG PET imaging in thyroid cancer- see PET tumor imaging section
REFERENCES:
(1) J Nucl Med 1998; Alexander C, et al. Intermediate and long-term side effects of
high-dose radioiodine therapy for thyroid carcinoma. 39: 1551-1554
(2) J Nucl Med 1994; Samuel AM, et al. Radioiodine therapy for well-differentiated thyroid cancer: a quantitative dosimetric evaluation for remnant thyroid ablation after surgery. 35: 1944-50
(3) Semin Nucl Med 1995; Dworkin HJ, et al. Advances in the management of patients with thyroid disease. 25: 205-220
(4) Endo Clin North Am 1990; Sept: p. 567-8
(5) J Nucl Med 1998; Muratet JP, et al. Influence of scanning doses of Iodine-131 on subsequent first ablative treatment outcome in patients operated on for differentiated thyroid carcinoma. 39: 1546-1550
(6) Endo Metab Clin North Am 1990; 19: 685-719
(7) Clin Nucl Med 1992; Park HM. Stunned thyroid after high-dose I-131 imaging. 17: 501-2 (No abstract available)
(8) Nuc Med Bio. 1986; Jeevanram. 13; 277,1986
(11) J Nucl Med 1992; Bushnell DL, et al. Complications, sequela and dosimetry of iodine-131 therapy for thyroid carcinoma. 33: 2214-21 (No abstract available)
(12) J Nucl Med 1994; Robinson PS, et al. Iodine-131 in breast milk following therapy for thyroid carcinoma. 35: 1797-1801
(13) J Nucl Med 1996; Hammami MM, Bakheet S. Radioiodine breast uptake in nonbreastfeeding women: clinical and scintigraphic characteristics. 37: 26-31
(14) Radiol Clin North Am 1993; Scott AM, Larson SM. Tumor imaging and therapy. 31: 859-79
(15) J Nucl Med 1995; Dottorini ME, et al. Assessment of female fertility and carcinogenesis after iodine-131 therapy for differentiated thyroid carcinoma 36: 21-27
(16) Semin Nucl Med 1995; Dworkin HJ, et al. Advances in the management of patients with thyroid disease. 25: 205-220
(17) J Nucl Med 1995; Dottorini ME, et al. Assessment of female fertility and carcinogenesis after iodine-131 therapy for differentiated thyroid carcinoma 36: 21-27
(18) J Nucl Med 1994; Pacini F, et al. Testicular function in patients with differentiated thyroid carcinoma treated with radioiodine.35: 1418-1422
(19) J Nucl Med 1992; Bushnell DL, et al. Complications, sequela and dosimetry of iodine-131 therapy for thyroid carcinoma. 33: 2214-21 (No abstract available)
(20) J Nucl Med 1994; Lubin E, et al. Serum thyroglobulin and iodine-131 whole-body scan in the diagnosis and assessment of treatment for metastatic differentiated thyroid carcinoma. 35: 257-262
(21) Thyroid 1994; Clark OH, et al. Managament of patients with differentiated thyroid cancer who have positive serum thyroglobulin levels and negative radioiodine scans. 4 (4): 501-505
(22) J Nucl Med 1988; Brendel AJ, et al. Thallium-201 imaging in the follow-up of differentiated thyroid carcinoma. 29: 1515-20
(23) J Nucl Med 2001; Schluter B, et al. Impact of FDG PET on patients with differentiated thyroid cancer who present with elevated thyroglobulin and negative 131-I scan. 42: 71-76
(24) J Nucl Med 2001; Macapinlac HA. Clinical usefulness of FDG PET in differentiated thyroid cancer. 42: 77-78 (No abstract available)
(25) J Nucl Med 2001; Coover LR, et al. Therapeutic 131-I in outpatients: A simplified method conforming to the code of federal regulations, Tital 10, Part 35.75. 41: 1868-1875
(26) J Nucl Med 2001; Solans R, et al. Salivary and lacrimal gland dysfunction (sicca syndrome) after radioiodine therapy. 42: 738-743
(27) J Nucl Med 2001; Sisson JC, Carey JE. Thyroid carcinoma with high levels of function: Treatment with 131I. 42: 975-983
(28) J Nucl Med 1998; Muratet JP, et al. Influence of scanning doses of iodine-131 on subsequent first ablative treatment outcome in patients operated on for differentiated thyroid carcinoma. 39: 1546-1550
(29) J Nucl Med 1994; Kim CK, et al. Influence of various scanning doses on subsequent I-131 ablation of thyroid remnants. 35:
(30) J Nucl Med 2001; Hermanska J, et al. Improved prediction of therapeutic absorbed doses of radioiodine in the treatment of thyroid carcinoma. 42: 1084-1090
(31) Eur J Nucl Med 2001; Petrich T, et al. Outcome after radioiodine therapy in 107 patients with differentiated thyroid carcinoma and initial bone metastases: side-effects and influence of age. 28: 203-208
(32) Acta Oncologica 1992; Hall P, et al. Tumors after radiotherapy for thyroid cancer. 31: 403-407
(33) Eur J Nucl Med 2001; de Keizer B, et al. Efficacy of high therapeutic doses of iodine-131 in patients with differentiated thyroid cancer and detectable serum thyroglobulin. 28: 198-202