Pulmonary Embolism:
View cases of pulmonary embolism
Clinical:
Pulmonary embolism (PE) is a frequently overlooked diagnosis which can be associated with significant mortality if untreated. The clinical diagnosis of pulmonary embolism is unreliable. Symptoms of PE include tachypnea/dyspnea (most common), tachycardia, hypoxia, pleuritic chest pain, hemoptysis, syncope, and atrial fibrillation. Massive PE may be associated with cor pulmonale and the ECG may show right axis deviation, P-pulmonale, RBBB, or other evidence of right heart strain. A normal arterial blood gas does not exclude the presence of a PE- in fact between 10-15% of patients with pulmonary embolism will have a pO2 over 85 mmHg. Similarly, a low arterial pO2 is non-specific. Unsuspected pulmonary embolism can be found in up to 1.5% of routine helical CT scans- the prevalence is higher in patients with underlying malignancy [24].
D-dimer is a cross-linked fibrin degradation product and a plasma marker of fibrin lysis. A serum level less than 500 ng/L excludes pulmonary embolism with a 90-95% accuracy [1]. Unfortunately, a positive test is non-specific (specificity is only 25-67%) and occurs in 40-69% of patients [1,41,62]. Additionally, the test is unreliable in the presence of malignancy, sepsis, recent surgery, or trauma [1]
Although PE occurs most commonly from deep venous thrombosis in the lower extremity, about 10% arise from clot in the upper extremity primarily associated with an indwelling catheter. In the PIOPED study, 92% of the patients with pulmonary embolism had at least one of the following risk factors: Immobilization, Recent Surgery, Underlying Malignancy, History of Deep Venous Thrombosis or Pulmonary Embolism, Estrogen use, or Pre-existing cardiac disease [2]. Only about 10% of cases of pulmonary embolism result in pulmonary infarction, due to the presence of the bronchial circulation [3].
Treatment for PE most commonly consists of anticoagulation with heparin or coumadin. Anticoagulation prevents clot propagation and allows endogenous fibrinolytic activity to dissolve existing thrombi [36]. Without anticoagulation therapy, PE has an estimated mortality rate of 30-36%, but with anticoagulation it has a mortality rate of 2.5% [30]. The risk of major hemorrhage with therapeutic heparin is 3-8% [30]. Other authors suggest that the risk of a major hemorrhagic complication from heparin therapy is actually closer to 1.8% [36].
Thrombolytic agents (such as urokinase or rtPA [recombinant tissue-type plasminogen activator]) are not routinely used for the treatment of acute PE. Clinical trials have demonstrated that thrombolytic therapy produces more rapid clot lysis and more rapid improvement in hemodynamics than anticoagulation therapy alone [36]. However, in hemodynamically stable patients no difference in patient mortality or the incidence of recurrent PE has been demonstrated [36]. Thrombolytic therapy is also associated with a higher risk for major hemorrhagic complications (12% incidence) and intracranial hemorrhage (1.2% incidence) [36]. Thrombolytic treatment is generally reserved for patients with massive pulmonary embolism producing circulatory shock (hypotension) [36]. Treatment may be useful in patients with evidence of right ventricular dysfunction [36]. Thrombolytic therapy is most effective when administered soon after PE and its effectiveness decreases with increasing symptom duration [36]. Presently, there is no data to support the intrapulmonary use of thrombolytic therapy [36].
For patients that cannot be anticoagulated, an inferior vena caval filter can be placed in order to prevent life-threatening PE. Major complications occur in about 1% of cases. Complications include central migration of the filter, filter fracture, inferior vena caval perforation, and vena caval thrombosis [48].
X-ray: View cases of pulmonary embolism
Ventilation-perfusion scintigraphy:
V/Q scanning has been the mainstay for screening symptomatic patients for the presence of pulmonary embolism. A negative V/Q scan essentially excludes PE, and a high probability study is associated with the presence of a PE in about 85% of cases at angiography. Confusion arises with low or intermediate probability examinations, and there is a 25%-35% disagreement among expert readers in the interpretation of scans in these categories. The problem with V/Q scanning is that it does not directly visualize thromboembolism, but rather its effects on perfusion and ventilation [47]. This problem causes the need for probability criteria, which in turn causes confusing results and high interobserver disagreement [47]. Nuclear medicine scanning for PE is probably most useful in previously healthy patients with a normal chest radiograph [38,46].
Plain film:
The CXR is abnormal in the majority of cases of PE. The PIOPED study showed that among patients with angiographically proven pulmonary embolism, only 12% had chest X-rays interpreted as normal [4] (24% of patients with PE in another study had normal CXR's [50]). Atelectasis and other focal pulmonary parenchymal abnormalities are the most common CXR findings in pulmonary embolism, occurring in up to 68% of patients with PE (cardiomegaly was the most common finding in another study [50]). Pleural effusions are also common (23% of patients in one study [50]), usually small, unilateral, and occupy less than 30% of the hemithorax [5]. Other palin film findings indicative of PE include regional oligemia (Westermark sign), a pleural-based wedge shaped area of increased opacity (Hampton's hump), and prominence of the central pulmonary artery (Fleischner sign) [22].
Angiography:
Patients are studied one lung at a time with an initial biplane (AP and lateral) run, followed by a contralateral oblique study. Contrast is 60% non-ionic injected at 25 cc per second for a total of 40 cc for each run. Angiography is actually a relatively safe procedure with a major complication rate of under 2%. Yet, at many institutions angiography is used in less than 15% of unresolved cases for pulmonary embolism [6]. Patients that should be considered to be at greater risk for complications include: intensive care unit patients, patients with tenuous right ventricular function (ie: pulmonary hypertension with pressure above 20 mmHg), and those with left bundle branch block (the procedure may produce a right bundle branch block and result in complete heart block). In patients with pulmonary arterial hypertension the necessity of the procedure should be reviewed or subselective injections only performed.
Classically, a embolus produces a filling defect within the affected pulmonary artery. Non-occlusive emboli have a "tram-track" appearance. Although considered the gold standard, angiography may not always detect the presence of emboli. Some indirect angiographic evidence for the presence of emboli such as vascular pruning and delayed capillary blush are non-specific. Additionally, agreement among angiographers regarding the presence of subsegmental emboli is poor (66% of cases in the PIOPED study [31]) and can be as low as 15%. V/Q scans can provide a road map to angiography, but if the abnormally perfused segment on the V/Q scan appears normal at angiography, complete evaluation the remainder of the lungs for the presence of pulmonary emboli is warranted. One important point to remember is that a negative angiogram has been shown to be an excellent indicator of a good prognosis [7].
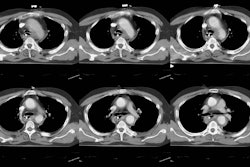
Helical CT:
General:
Up to 30% of patients evaluated for PE will have intermediate probability V/Q scans and negative lower extremity duplex exams for deep venous thrombosis [19]. Ultimately, between 20-30% of these patients will be shown to have PE at angiography. Unfortunately, despite the relative safety of pulmonary angiography, many physicians are reluctant to proceed to this step. With advances in helical and electron beam CT the role of computed tomography in the diagnosis of PE may be changing. Helical CT is able to identify main, lobar, and segmental emboli with a reported sensitivity over 90%. Emboli as small as 2 mm in the 7th order vascular divisions have been detected. Although the detection of subsegmental emboli is worse, the clinical significance of these small emboli has not yet been establishished. Additionally, on angiography there is poor interobserver agreement for the presence of subsegmental emboli [31] and the true incidence of isolated subsegmental emboli is difficult to determine. Helical CT has also been shown to have a significantly better sensitivity, specificity, positive, and negative predictive values compared to V/Q scanning [44]. Helical CT permits a more confident diagnosis to be made in a greater number of cases when compared with V/Q scanning [44].
It has been suggested that helical or electron beam CT should be the initial imaging modality to screen patients suspected of having PE- particularly in patients with abnormal CXR's in whom there is a greater likelihood of inconclusive V/Q scan results [46].. If emboli are detected, no further work-up is required. If this study is negative, a lower extremity US or CT DVT exam can be performed to assess for the presence of DVT. Again, a positive exam would lead to patient treatment. If both studies were negative, then a decision would be required regarding whether the patient should proceed to angiography or not [1].
Another benefit of CT is the ability to suggest an alternative diagnosis in 11-57% of patients to explain their clinical symptoms [19,23,32,37]. However, the use of helical CT in the diagnosis of pulmonary embolism is still open to debate. Some authors have questioned the utility of helical CT as a first line imaging study for the diagnosis of PE [27,41] and the safety of withholding anticoagulation if the CT exam is negative [41]. Although initial data indicate a good clinical outcome for patients with negative CT PE exams [44,46], a closely scutinized, prospective, controlled clinical trial has not yet been performed. It is probably best to think of CT as a SCREENING exam and not necessarily a "gold standard" test. When thin section CT PE images are coupled with a CT DVT exam the study probably provides an excellent survey for clot. None-the-less, further evaluation for pulmonary embolism in selected cases following negative CT exams may be reasonable in selected cases. Although there is obvious utility for helical CT in the work-up of suspected pulmonary embolism- individuals need to make their own assessment of how CT should be employed.
Single Detector Helical CT exam:
Many different approaches to helical CT scanning for the detection of PE have been described, however, at the present time neither the exam nor the contrast concentration/injection rate have been standardized. The exam described below is mostly taken from that described by Dr. Remy-Jardin [17].
A conventional noncontrast CT scan of the chest may be performed first. This can be done utilizing narrow collimation and 15 mm intervals or with a standard helical exam. This exam will permit the detection of parenchymal abnormalities (infarction) or pleural effusion, identify calcified hilar lymph nodes, and enables localization of the anatomic volume of interest. The use of narrow collimation can aid in detecting subtle parenchymal or bronchial changes associated with unsuspected chronic pulmonary embolism and will also help to limit radiation dose.
The contrast exam should be performed from the level of the aortic arch to approximately 2 cm below the level of the inferior pulmonary veins with the patient scanned at end inspiration [37]. Hyperventilation before the start of the exam (consisting of a few deep breaths) is recommended as it will facilitate prolonged breath holding. Patients unable to maintain a breath hold can be scanned while gently breathing to reduce respiratory motion artifacts.
The scan is performed from just above the lower hemidiaphragm to the aortic arch (caudocranial direction) during an infusion of 150-200 ml of 30% contrast material (3-5 ml/sec) with a 15-20 sec scan delay (some centers use 60% iodinated contrast and a lower injection rate). Alternatively, a small test injection (18 cc) can be used to determine optimal timing of the scan. The time to peak enhancement plus 5 seconds is used as the time delay for the diagnostic scan. The extra 5 seconds allows for opacification of the distal small arteries and provides a margin for error. For patients with right ventricular failure or pulmonary hypertension a longer scan delay is required (15-18 seconds) [37]. Scanning caudo to cranial and using a lower injection rate reduces streak artifacts from concentrated contrast material in the superior vena cava which may obscure the adjacent right main pulmonary artery. Also, caudo-cranial imaging will minimize motion artifacts due to respiration that tend to be greatest in the lung bases and less significant in the upper lungs.
A 3 mm collimation, table feed of 5 mm/sec (pitch of 1.7), 240-300 mA, with a 20 to 30 second exposure, and 1.5 mm overlapping reconstruction images read at the workstation will provide excellent image quality [16]. Patients not able to hold their breath for 20 to 30 seconds can be imaged with shorter scan times (10 mm/sec table feed with 5 mm collimation and a 10 second breath hold [pitch of 2]), but there is a decrease in spatial resolution [17]. Patients on a respirator can be imaged during a forced period of apnea or while breathing at a minimal tidal volume and respiratory rate [18].
For sub-second scanners, improved evaluation of subsegmental arteries can be obtained with the use of 2 mm collimation, 0.75 sec per revolution, and a pitch of 2 (ie: table speed of 4 mm per sec) [8,37]. Overlapping reconstruction images are not required when using this narrow collimation [37]. Electron beam CT has minor advantages in analyzing paracardiac arteries due to a reduction in motion artifact and higher contrast enhancement , but the images suffer from slightly increased noise [42].
Images should be reconstructed using a 180 degree linear interpolation algorithm to decrease partial volume effects [37].
NOTE: A womans breasts recieve between 2.0-3.5 rad of radiation from a thoracic CT exam. It should be kept in mind that delivery of 1 rad of radiation to a womans breasts before age 35 years fractionally increases her risk of breast cancer by 13.6% over the expected spontaneous rate for the general population [37].
Multi-detector Helical CT exam:
Multi-detector helical CT is superior to the single detector examination for the evaluation of sub-segmental pulmonary arteries [59]. Up to 74% of fifth-order arteries can be identified when using a section thickness of 1.25 mm [59]. The main reason for inadequate detection of these small vessels is partial volume effects, and cardiac and respiratory motion [59].
Sensitivity/Specificity/Prognosis:
Single detector helical CT has a reported sensitivity for pulmonary embolism in a segmental artery or larger of between 53% to 100% (helical CT has a higher sensitivity than V-Q scintigraphy [37]). Some of the lower sensitivity data reported is from early studies in which thicker collimation (5mm) was utilized. Specificity is between 78 to 96%. Negative predictive value is 81-100% and positive predictive value is 60-100% for detecting emboli within the central pulmonary arteries [See references below]. The negative predictive value of a normal exam is variable. Pulmonary embolism has been reported in 5.4% to 15% of patients with normal helical CT scans [19,23,27]. This is higher than the 0.6% to 4.2% incidence of PE in patients with negative pulmonary arteriograms [19]. Dual-section helical CT is superior to conventional helical CT in the detection of pulmonary embolism- particularly at the subsegmental level [53].
Although sensitivity and specificity data is important, one also needs to address the clinical outcome following a negative exam. Patients with negative helical CT exams have been shown to do well clinically [34,43,49]. In one retrospective study, no patient with a negative helical CT for PE subsequently developed an embolism and there were no patients deaths attributable to PE during a 6 month follow-up period [43]. Other studies have shown subsequent thromboembolic events in 3-5% of patients with negative CT PE exams [44,49]. A recent prospective study found a 1% incidence of subsequent pulmonary embolism in patients with negative helical CT PE exams [46]. These preliminary data indicate a favorable outcome for patients with negative helical CT PE exams. The likelihood for subsequent pulmonary embolism may even be less if the CT PE exam is coupled with a negative CT DVT exam.
Although sensitivity is better for detection of central emboli, when subsegmental vessels are included, sensitivity decreases to 63-67% for ceonventional helical CT, while specificity ranges from 78-100% [9,11,23]. Theoretically, this loss of sensitivity should affect only those patients with isolated subsegmental emboli. Unfortunately, interobserver agreement among angiographers as to the presence of subsegmental emboli is poor [28]. The reported incidence of isolated sub-segmental emboli ranges from 4%, to as high as 36% in patients referred for angiography [9,10,13,28,30,45,53]. In the PIOPED study, 5.6% of patients enrolled in the study and 16% of patients with positive angiographic findings had isolated subsegmental pulmonary emboli (for patients with low probability scans, the incidence was 17%). To further complicate matters, the clinical significance of subsegmental emboli has not been fully described. Patients with untreated isolated subsegmental emboli may not be at increased risk for a poor clinical outcome [28] and in one study only 1 of 78 patients (1.2%) with a negative helical CT subsequently developed microemboli (detected at autopsy) [34]. However, small emboli may produce significant morbidity in patients with underlying cardiorespiratory disease [23,28]. Additionally, among stable patients small emboli may indicate a risk for recurrent more significant emboli. In these cases, angiography may still be required despite a negative helical CT exam. Improved evaluation of subsegmental arteries can be obtained by imaging with the use of 2 mm collimation, 0.75 sec per revolution, and a pitch of 2 (4 mm per revolution table feed) [8].
The utility of helical CT in the evaluation of critically ill patients has also been questioned [60]. A lower sensitivity and specificity for detection of PE in a subgroup of critically ill surgical trauma patients has been suggested- possibly related to extensive underlying parenchymal abnormalities. Unfortunately, this study suffered from a very small sample size and no effort was made to suspend respiration during the examination which likely resulted in degradation of image quality [60].
Image interpretation:
Images should be reviewed on a workstation. The signs of PE include a central intravascular filling defect outlined by contrast material within the vessel lumen, eccentric tracking of contrast material around a filling defect, and complete vascular occlusion. Filling defects that form a smooth, obtuse angle with the vessel wall usually represent chronic thrombi, but may represent the sequella of recent resolving emboli. The lung parenchyma distal to the thrombus may be oligemic- demonstrating a decreased number and caliber of vessels, and decreased attenuation.
Although no pleuroparenchymal findings are noted in about 30% of cases of acute PE [35], parenchymal abnormalities are commonly detected [33,35]. Findings include pulmonary hemorrhage, which appears as an area of ground-glass attenuation or air-space consolidation (indistinguishable from edema or pneumonia), and pulmonary infarction (occurs in 10% of cases). Infarction classically appears as a peripheral wedge-shaped, pleural-based opacification with its apex directed towards the hilum. Wedge shaped opacities can be found in 25-67% of patients with PE, but the finding can also occur in patients without embolism [33,35]. There may be a thickened vessel identified entering at the apex of the opacification ("vascular sign") and this can help distinguish an infarct from an area of pneumonia [35]. Infarcts may have a truncated apex if the lobules immediately subtended by the embolus have adequate collateral bronchial circulation. Hemorrhage without infarction usually resolves within a week, while infarcts decrease slowly in size over 3 to 5 weeks. The infarct may resolve completely, or leave a fibrous scar with associated pleural thickening. True cavitation is unusual with bland infarction and typically implies secondary infection of the necrotic tissue or septic emboli. Pleural effusions are a common feature of PE (roughly 50% of cases), but the finding is not specific as it can also be seen in patients without PE [33,35]. Effusions develop almost immediately, are usually small and unilateral, and are often hemorrhagic [3].
On follow-up exams performed 6 weeks after acute pulmonary embolism, complete resolution of thrombus is found in only 32% of patients (most commonly in patients with low initial clot burdens). The majority of patients demonstrate some clot resolution and some abnormalities such as eccentric emboli contiguous with the vessel wall or filling defects with central contrast material representing recanalization. This is important to note, as these findings were previously felt to represent changes of chronic thromboembolic disease [25].
Pitfalls in CT imaging for PE:
Limitations of the procedure include patient inability to comply with the exam secondary to dyspnea and poor vascular opacification. Technically inadequate exams (mainly the result of patient dyspnea) have been reported in 1 to 10% of cases and a technically adequate exam may still be considered inconclusive in up to 9% of cases [9]. Respiratory motion can result in a degree of limitation in exam interpretation in up to 27% of cases [54].
Interobserver agreement for helical CT is better than that for V/Q scanning [44]. Interobserver agreement for clot within lobar arteries is very good [49], but agreement decreases with more peripheral vessels [29]. Obliquely/horizontally oriented vessels within the right middle lobe and left lingular region make detection of emboli in these sites difficult [23], but isolated emboli to these vessels would be rare (2.5% in one study [10]). Anterior segmental arteries of the upper lobes and the superior segmental arteries of the lower lobes also run obliquely. Angled 2D reformations through the obliquely oriented vessels may be useful in excluding PE [12], but may not be necessary with the use of smaller collimation (2mm) [17].
Intersegmental lymph nodes may be misinterpreted as clots- viewing images so that vessels are displayed along their long axis helps to decrease this artifact. Breathing artifact or cardiac motion may also produce false positive exams (ie: pseudoarterial filling defects) [9,26]. Review of lung parenchymal windows should reveal other evidence of respiratory motion which may not be evident on mediastinal window settings. A right to left shunt (patent foramen ovale) results in a lower degree of pulmonary artery enhancement which can reduce exam quality [17]. Unilateral extensive airspace consolidation can result in ipsilateral increased vascular resistance with the resultant slow flow producing spurious filling defects within the pulmonary arteries that can be mistaken for emboli [17,37]. Patients with CHF may have a circumferential collar of low attenuation around a proximal segmental artery secondary to perivascular edema [17].
Flow related central regions of decreased attenuation may be identified and mistaken for clot on studies performed during the terminal phases of contrast injection. This artifact is suspected to result from laminar flow of the contrast media. There is more rapid inflow of unopacified blood into the central portion of the vessel, while opacified blood persists peripherally due to slower flow near the vessel wall [24].
Combined helical CT for exclusion of PE and DVT:
Because more than 90% of pulmonary emboli arise from the deep veins of the legs or pelvis [58], the use of helical CT for the exclusion of deep venous thrombosis during imaging for pulmonary embolism has also been explored [20,21,39,40,51,54]. This type of combined CT PE/DVT exam allows for "one-stop" evaluation of patients with suspected pulmonary embolism. The CT examination can easily identify clot within the deep venous system of the lower extremities. CT can also identify clot within the pelvic veins or IVC which are not well assessed by ultrasound (the pelvic veins or IVC can be the source for pulmonary embolism in 4% to 11% of cases of PE [51,58]).
Venous enhancement persists for some time following the intravenous injection of contrast material which permits detection of clot within the venous structures [21]. Ideally, scanning should be performed during the period of peak venous enhancement. Venous enhancement increases slowly and has a gradual decline [40]. A time delay of approximately 120 seconds following completion of the CT PA exam allows for good venous enhancement in the majority of patients [40]- this is roughly 3 minutes following initiation of the contrast injection [57]. Only about 1-2% of examinations for DVT are inconclusive- usually due to poor venous enhancement or streak artifacts from orthopedic hardware or dense arterial calcifications [51,56], however, up to 16% of exams may have segments of the deep venous system which cannot be accurately assessed (ie: due to beam hardening artifacts associated with orthopedic hardware, etc.) [56].
NOTE: Acute thrombi less than 8 days old have an average attenuation value of 66HU. Thrombi older than 8 days have an average attenuation of 55 HU [40]. Intravenous enhancement should optimally exceed these levels for clot detection.
No standard protocol has been established for this procedure. A suggested protocol calls for axial 5 mm scans performed at 5 cm intervals from the upper calves to the diaphragm [39,58]. Although most leg clots are long segment, a 5 cm gap between slices will result in the failure to identify all cases of deep venous thrombosis [58]. Some authors have concluded that using a slice gap of greater than 2 cm can potentially lead to false negative findings on CT or an underestimation of the extent of clot [56]. Another protocol calls for 1 cm images (pitch of 1) performed from the iliac wings to the tibial plateau [21,51]. If using this continuous helical acquisition, one must consider the large quantity of images generated and the additional radiation dose to the patient. At my institution, we are presently using a protocol that calls for 5 mm thick images performed at a 10 mm gap from the popliteal fossa to the mid abdomen. Further investigations need to be performed to determine the optimal protocol.
Indirect CT venography can identify DVT in 4-5% of patients with a negative CT PE exam [51,58]. The finding of DVT in these patients obviously results in a change in patient management. The sensitivity and specificity of helical CT for the detection of femoral popliteal DVT is compable to lower extremity US [51,52]- sensitivity 89-97%, specificity 94-100%, and accuracy 93% [54,55,58]. There are cases in which the CT exam of the lower extremities may be positive and the US exam negative (excluding pelvic vessels and the IVC)- possibly due to flow artifacts or volume averaging with valves [51,52,54]. False positive CT exams typically involve a subtle finding seen on only a single image [54]. It has been recommended that US be used to confirm isloated DVT identified on CT prior to initiation of anticoagulation [54]. Of course, because there is always a time delay between the two exams, one can never be absolutely certain that these cases don't actually represent false negative ultrasounds [51]. Extensive, bilateral lower extremity thrombosis may also not be properly identified at CT [54]. In cases of bilateral, extensive DVT findings which should suggest the diagnosis include prolonged arterial phase enhancement, venous dilatation, and vessel wall enahncement [54]. Flow artifacts can be reduced in patients with suspected abnormal hemodynamic status by increasing the delay prior to the DVT portion of the exam to 4 minutes post injection [52]. Interobserver disagreement can occur in up to 12% of cases [56].
MRI:
On MRI, using standard or gated spin echo examinations, pulmonary emboli appear as foci of increased signal intensity within the signal void of the pulmonary artery lumen. Occasionally, blood within the vessels may demonstrate high signal- usually this occurs during the diastolic portion of the gated exam. By obtaining multiple acquisitions gated to varying points in the cardiac cycle (each 100 msec apart) a sequence of images can be obtained and the increased signal associated with slow flow will disappear on at least one image. Patients with pulmonary hypertension, however, may have markedly diminished flow velocities and even during systole the increased signal within the vessel may remain. This finding was observed in all patients with pulmonary systolic pressures greater than 80 mm Hg [14].
It is now more common to evaluate the pulmonary circulation using MR angiography during suspended respiration following the intravenous administration of gadolinium. MR has been shown to have a sensitivity as high as 85%, and a specificity of 96% [30]. MR is not adequate for the detection of subsegmental emboli [30].
Transthoracic/Transesophageal Echocardiography:
The one advantage of these studies is that they can be performed at the patients bedside, however, in comparison to helical CT, transthoracic and transesophgeal echo have limited accuracy for detecting pulmonary embolism. The central pulmonary arteries can be visualized by transesophageal echo and in one study, the sensitivity for central PE was 82%. Unfortunately, in order to detect more peripheral PE these procedures rely on indirect evidence of PE including tricuspid regurge, RV dilatation, paradoxical wall motion, and widening of the pulmonary artery diameter. Overall sensitivity in one study was 59%, with a 77% overall specificity [15]. Right ventricular hypokinesis detected by echocardiography is an important predictor of mortality associated with acute pulmonary embolism [50].
REFERENCES:
(1) Radiology 1996; Diagnosis of acute pulmonary embolism: time for a new approach 199 (1): 25-27 (No abstract available)
(2) Radiol Clin North Am 1993; 31(4): 849-858
(4) Radiology 1993;189:133-136
(5) Am Rev Resp Dis 1978; 177: 829-834
(6) RadioGraphics 1997; CT of acute pulmonary emboli: where does it fit? 17: 1037-1042 (No abstract available)
(7) Radiology 1978; 126:561-567
(8) Radiology 1997; 204: 157-163
(9) Radiology 1996; 200: 699-706
(10) Radiology 1996; 199: 31-35
(13) Radiology 1997; Pulmonary arteries must be seen before they can be assessed.204: 11-12 (No abstract available)
(14) AJR 1987; 149: 15-21
(16) J Thorac Image 1997; Reply to opinions.12: 100-102 (No abstract available)
(17) J Thorac Image 1997; 12: 103-117
(18) AJR 1997; CT Evaluation of pulmonary embolism: Technique and interpretation. 169: 959-965 (No abstract available)
(19) Radiology 1997; 205: 453-458
(20) AJR 1998; Loud PA, et al. Combined CT venography and pulmonary angiography: A new diagnostic technique for suspected thromboembolic disease. 170: 951-954 (No abstract available)
(21) ARRS 98th Annual Meeting Abstract Book 1998; Yankelevitz DF, et al. Technical considerations for combining pulmonary CT angiography with lower extremity CT venography. 63-63
(22) Radiology 1998. 207: 753-758
(23) Radiology 1998; 208: 201-208
(24) Radiology 1998; 208: 209-215
(25) J Comput Assist Tomogr 1998; Van Rossum AB, et al. Spiral CT appearance of resolving clots at 6 week follow-up after acute pulmonary embolism. 23 (3): 413-417
(26) AJR 1998; Beigelman C, et al. Pitfalls in diagnosis of pulmonary embolism with helical CT angiography. 171: 579-585 (No abstract available)
(27) Radiology 1998; Drucker EA, et al. Acute pulmonary embolism: Assessment of helical CT for diagnosis. 209: 235-241
(28) AJR 1998; Diffin DC, et al. Effect of anatomic distribution of pulmonary emboli on interobserver agreement in the interpretation of pulmonary angiography. 171: 1085-1089
(29) AJR 1999; Chartrand-Lefebvre C, et al. Contrast-enhanced helical CT for pulmonary embolism detection: inter- and Intraobserver agreement among radiologists with variable experience. 172: 107-112.
(30) Radiology 1999; Gupta A, et al. Acute pulmonary embolism: Diagnosis with MR angiography. 210: 353-359
(31) Radiology 1999; Stein PD, et al. Reassessment of pulmonary angiography for the diagnosis of pulmonary embolism: Relation of interpreter agreement to the order of the involved pulmonary arterial branch. 210: 689-691
(32) Radiology 1999; Kim KI, et al. Clinically suspected pulmonary embolism: Utility of spiral CT. 210: 693-697
(33) Radiology 1999; Shah AA, et al. Parenchymal and pleural findings in patients with and patients without acute pulmonary embolism detected at spiral CT. 211: 147-153
(34) AJR 1999; Garg K, et al. Clinical validity of helical CT being interpreted as negative for pulmonary embolism: Implications for patient treatment. 172: 1627-1631
(35) J Comput Assist Tomogr 1999; Johnson PT, et al. Spiral CT of acute pulmonary thromboembolism: Evaluation of pleuroparenchymal abnormalities. 23 (3): 369-373
(36) Chest 1999; Arcasoy SM, Kreit JW. Thrombolytic therapy of pulmonary embolism. A comprehensive review of current evidence. 115: 1695-1707
(37) Radiology 1999; Remy-Jardin M, Remy J. Spiral CT angiography of the pulmonary circulation. 212: 615-636
(38) Radiology 1999; Novelline RA, et al. Helical CT in emergency radiology. 213: 321-339
(39) AJR 2000; Loud PA, et al. Combined CT venography and pulmonary angiography in suspected thromboembolic disease: Diagnostic accuracy for deep venous evasluation. 174: 61-65
(40) AJR 2000; Yankelevitz DF, et al. Optimization of combined CT pulmonary angiography with lower extremity CT venography. 174: 67-69
(41) Ann Intern Med 2000; Rathbun SW, et al. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: A systematic review. 132: 227-232
(42) Radiology 2000; Schoepf UJ, et al. Segmental and subsegmental pulmonary arteries: Evaluation with electron-beam versus spiral CT. 214: 433-439
(43) J Vasc Interv Radiol 1999; Lomis NN, et al. Clinical outcomes of patients after a negative spiral CT pulmonary arteriogram in the evaluation of acute pulmonary embolism. 10: 707-712
(44) AJR 2000; Blachere H, et al. Pulmonary embolism revealed on helical CT angiography: Comparison with ventilation-perfusion radionuclide lung scanning. 174: 1041-1047
(45) Radiology 2000; de Monye W, et al. Suspected pulmonary embolism: Prevalence and anatomic distribution in 487 consecutive patients. 215: 184-188
(46) Radiology 2000; Goodman LR, et al. Subsequent pulmonary embolism: Risk after a negative helical CT pulmonary angiogram- prospective comparison with scintigraphy. 215: 535-542
(47) AJR 2000; Smith TP. Pulmonary embolism: What's wrong with this diagnosis? 174: 1489-1497 (Review. No abstract available)
(48) Radiology 2000; Athanasoulis CA, et al. Inferior vena caval filters: Review of a 26-year single center clinical experience. 216: 54-66
(49) AJR 2000; Remy-Jardin M, et al. Clinical value of thin collimation in the diagnostic workup of pulmonary embolism. 175: 407-411
(50) Chest 2000; Elliott CG, et al. Chest radiographs in acute pulmonary embolism. Results from the international cooperative pulmonary embolism registry. 118: 33-38
(51) Radiology 2000; Cham MD, et al. Deep venous thrombosis: Detection by using indirect CT venography. 216: 744-751
(52) AJR 2000; Garg K, et al. Thromboembolic disease: Comparison of combined CT pulmonary angiography and venography with bilateral leg sonography in 70 patients. 175: 997-1001
(53) Radiology 2000; Qanadli SD, et al. Pulmonary embolism detection: Prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. 217: 447-455
(54) AJR 2000; Duwe KM, et al. Evaluation of the lower extremity veins in patients with suspected pulmonary embolism: A retrospective comparison of helical CT venography and sonography. 175: 1525-1531
(55) AJR 2001; Coche EE, et al. Using dual-detector helical CT angiography to detect deep venous thrombosis in patients with suspicion of pulmonary embolism: diagnostic value and additional findings. 176: 1035-1039
(56) AJR 2001; Garg K, et al. Thromboembolic disease: Variability of interobserver agreement in the interpretation of CT venography with CT pulmonary angiography. 176: 1043-1047
(57) AJR 2001; Bruce D, et al. Combined CT venography and pulmonary angiography: How much venous enhancement is routinely obtained? 176: 1281-1285
(58) Radiology 2001; Loud PA, et al. Deep venous thrombosis with suspected pulmonary embolism: Detection with combined CT venography and pulmonary angiography. 219: 498-502
(59) Radiology 2001; Ghaye B, et al. Peripheral pulmonary arteries: How far in the lung does multi-detector row spiral CT allow analysis? 219: 629-636
(60) Arch Surg 2001; Velmahos GC, et al. Spiral computed tomography for the diagnosis of pulmonary embolism in critically ill surgical patients. A comparison with pulmonary angiography. 136: 505-510
(61) Arch Intern Med 2001; Goldstein NM, et al. The impact of the introduction of a rapid D-dimer assay on the diagnostic evaluation of suspected pulmonary embolism. 161: 567-571
(62) JAMA 2001; Kline JA, et al. Diagnostic accuracy of a bedside D-dimer assay and alveolar dead-space measurement for rapid exclusion of pulmonary embolism. A mutlicenter study. 285: 761-768