The medical imaging community should be neither alarmed nor develop a gadolinium phobia while waiting for guidance from regulators over controversial gadolinium contrast agents. This is the opinion of Dr. Howard Rowley, the Joseph Sackett Professor of Radiology at the University of Wisconsin in the U.S. and president of the American Society of Neuroradiology.
"There is understandable concern, but gadolinium is, and should be, expected to deposit in various tissues in the body and, based on what we know about other metals, it's not a case for alarm. It doesn't necessarily imply a toxic effect," he told ECR Today in advance of the congress.
Other metals critical for normal physiology also have been found to deposit in the body, and this can provide insight into the "imaging curiosity" seen with gadolinium, he explained. Animal and human autopsies have shown, to date, no tissue injury at the sites where gadolinium deposits, he added. "At the levels of tissue deposition we're seeing, it's not toxic, and there are no symptoms associated with them."
Rowley is concerned the radiology community was drawing parallels between gadolinium deposition and nephrogenic systemic fibrosis (NSF), which is unrelated. Such parallels are unfounded, he continued. "The important point is this is different to NSF, which can cause tissue injury and harm. But we shouldn't infer similar mechanisms in the body between the two."
The debate has become heated as unanswered questions abound and confusion reigns, with pressure on regulators to decide a position on contrast agents. Overall, the risk/benefit ratio was still largely in favor of using gadolinium, but Rowley acknowledged more research and observation were required, adding there is cause for caution but not alarm.
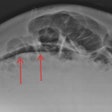
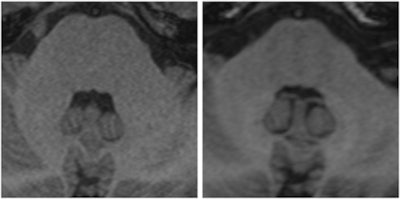 Two images of a patient prior to (left) and after (right) nine injections of the linear gadolinium-based contrast agent Magnevist. The image prior to injection shows no hyperintensities displayed in the dentate nucleus, while a clear signal intensity increase in the dentate nucleus becomes visible after nine injections of Magnevist. Images courtesy of Dr. Alexander Radbruch, head of neuro-oncologic imaging at the University Hospital Heidelberg in Germany.
Two images of a patient prior to (left) and after (right) nine injections of the linear gadolinium-based contrast agent Magnevist. The image prior to injection shows no hyperintensities displayed in the dentate nucleus, while a clear signal intensity increase in the dentate nucleus becomes visible after nine injections of Magnevist. Images courtesy of Dr. Alexander Radbruch, head of neuro-oncologic imaging at the University Hospital Heidelberg in Germany.At today's session, he intends to speak about the preclinical data around the recent discovery that gadolinium deposits in the body -- a finding that has rattled radiologists.
Gadolinium is a toxic heavy metal not normally found in the body, but when chelated to reduce toxicity, it becomes a powerful contrast agent for MRI scans and has vastly improved the imaging of body organs and tissues. It has been used successfully for years, with eight agents approved for use. However, the element has found itself in the middle of a scientific controversy after it was discovered that patients with renal failure who had been administered gadolinium for scans were at increased risk of developing the condition NSF, which involves the thickening and scarring of the skin and internal organs.
Further research has since revealed that some of the agents can undergo dechelation in patients with normal renal function, resulting in gadolinium deposits in the brain and other body tissue. The findings have shocked the imaging community -- what had been go-to contrast agents are now being viewed with some caution. With studies thin on the ground and numerous unanswered questions, radiologists have been left confused over how to approach the eight approved agents. Both the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) are expected to clarify the situation and provide guidelines for use in 2017.
The issue has become controversial for two main reasons, said Dr. Val Runge, professor of radiology and physician at Bern University Hospital in Switzerland. First, he said, "At this time, there are no definitive, proven associated symptoms," despite several papers suggesting symptoms associated with gadolinium deposition. Second, the radiology community is arguing over which of the approved agents are implicated in gadolinium deposition and how they should be treated by the regulators.
The agents can be classified into three general groups, Runge explained: the nonionic linear agents (Omniscan and OptiMark), which are more likely to undergo dechelation and have been implicated in gadolinium deposition; the ionic linear agents with medium stability (Magnevist, MultiHance, Primovist); and the macrocyclic agents (Dotarem, Gadavist, ProHance), which are the most stable and least likely to dechelate.
"The regulators could do one of three things," he explained. "They could do nothing -- say it's too early and recommend more studies. They could take an intermediate position where they put restrictions on certain agents. Or they could remove some agents from the market. In Europe, the EMA is likely to pursue one of the two latter courses."
The problem is there is no easy answer, he said. For example, where do the regulators draw the line between agents if they were to place restrictions, and, were they to go for the third option, how would they decide which agents to remove and in what time frame? Furthermore, could the manufacturers of the remaining agents increase supply to meet the shortfall in the market?
"These are not insignificant problems," said Runge, who will review the published clinical papers in today's session. "The question most likely comes back to the agents in the middle -- should they be withdrawn or be limited? This is an ongoing discussion by medical authorities across the world."
Rowley, however, is of a different mindset, believing it is unhelpful to segregate the different agents into groups based on the strength of chelation. Instead, he suggested that radiologists view all agents as sources of deposition and consider all variables and specific applications when choosing which agent to use.
"It would be premature to conclude one agent is better or safer over another. I don't think the data are there yet," he said.
Regulators are faced with a tough decision. In the meantime, radiologists will be waiting. Today's session should provide clarity on the current landscape and thinking on gadolinium-based contrast agents with an overview of the research to date, as well as a discussion on dealing with the challenges facing radiologists using these contrast agents.
Originally published in ECR Today on 3 March 2017.
Copyright © 2017 European Society of Radiology