CNS Trauma:
Tc99m-HMPAO Imaging in CNS Trauma:
Patients with mild traumatic brain injury (TBI) experience pyschologic and cognitive problems acutely after the injury including cognitive slowing, poor concentration, attention difficulties, fatigue, and impaired memory [9]. In most patients, these symptoms gradually resolve over time, however, about 10% of patients remain symptomatic at one year and subtle abnormalities may persist for months or years [9]. Also, within the first year after head trauma the incidence of seizures exceeds the general population risk for the development of epilepsy by 12 fold. The latency period between head injury and the development of seizures varies, but close to 60% of patients will experience the onset of seizures within 1 year of the injury.
Tc99m-HMPAO imaging is more sensitive than CT or MRI in detecting post traumatic CNS abnormalities both in the acute [5] and remote stages. In the setting of acute trauma, small, non-focal frontal or occipital defects are associated with a favorable prognosis. Large or multifocal defects involving the parietal or temporal lobes, the cerebellum, or the brainstem are associated with an unfavorable prognosis.
Brain Tumors:
Tc99m-HMPAO Imaging in Brain Tumors
(See also Thallium Imaging in CNS Tumors)
Viable areas within CNS neoplasms are generally expected to demonstrate increased metabolism, while areas of necrosis show no metabolic activity. Increased activity within certain lesions is likely related to increased flow to the lesion given that Tc99m-HMPAO is a perfusion agent. There is generally increased uptake of the tracer in meningiomas when compared to gliomas (the exception to this is calcified meningiomas which demonstrate decreased uptake). Within gliomas, regional tracer uptake increases in relationship to the grade of malignancy [5], with low grade (I and II) lesions demonstrating less uptake than grade III tumors. Grade I and II lesions typically demonstrate uptake less than the cerebellum. Uptake within grade IV tumors tends to be mixed (high and low uptake zones) due to the presence of necrosis within the lesion. Uptake within primary brain tumors tends to correlate with the level of glutathione within the tumor [1]. Metastatic CNS neoplasms generally demonstrate decreased tracer uptake, the exception to this being renal cell carcinoma. Post-op gliosis can demonstrate increased Tc99m-HMPAO accumulation compared to the surrounding nonaffected brain tissue, although the uptake is generally not greater than that in the cerebellum. [1]
123 I-iodomethyltyrosine:
123 I-iodomethyltyrosine (IMT) is an amino acid agent (amino acid transport is increased in malignant lesions) [4]. IMT is rapidly taken up in brain tumors with a good target-to-background ratio [4]. Approximately 15-30 minutes after injection, uptake peaks. The agent is not incorporated into protein, and slowly washes out of the tumor (about 30% washout at 1 hour after injection) [4]. The sensitivity is very high for high-grade CNS neoplasms, but decreases for low grade lesions [4].
CNS Infection:
Tc99m-HMPAO Imaging in CNS Infection:
Viral Encephalitis (Herpes encephalitis): During the acute phase of a viral encepalitis the Tc99m-HMPAO exam typically demonstrates an area of increased perfusion (i.e.: a `hot spot') in up to 94% of cases. During the subacute phase (15 days after presentation) a followup exam may demonstrate either normal or decreased tracer uptake at the site of infection. Patients with a normal perfusion pattern during the subacute phase have a very good clinical prognosis. The decreased perfusion pattern, however, is associated with decreased intelligence or learning disabilities [2]. Discordant increased Tc-HMPAO activity, but decreased activity on Tc-ECD exam has been reported [3].
SPECT Imaging in Lyme Encephalopathy:
Lyme disease is a multisystem illness caused by the tick-borne spirochete Borrelia burgdorferi. The disease has 3 stages- stage 1 occurs 2-30 days after the tick bite with flu-like symptoms and an expanding skin lesion (erythema chronicum migrans) [10]. Stage 2 presents 1-4 months after infection and consists of cardiac and neurologic symptoms [10]. Stage 3 occurs up to a few years later and manifests as arthritis and chronic neurologic symptoms [10]. The stages may overlap or may occur alone [10]. CNS infection produces a variety of neurologic and psychiatric disturbances [7]. Patients can experience short term memory loss, severe depression (seen in up to 70% of patients), and personality changes marked by irritability and mood swings [8]. The triad of meningitis, cranial neuritis, and radiculoneuritis is characteristic of neurolyme [10]. Facial palsy, including bilaterak facial palsy, is common in neuro-lyme disease [10].
Patients with Lyme encephalopathy generally show a poor response to medications that would ordinarily be helpful and the patient's psychiatric findings can improve with antibiotic treatment alone [8]. Lyme encephalopathy most commonly produces a multifocal pattern of hypoperfusion affecting both the cortex and deep structures [6,8]. Significant perfusion abnormalities can be identified in up to 50% of affected patients [8]. Diffusely reduced cerebral cortical flow has also been described [7]. SPECT imaging can also be used to monitor response to therapy as areas of abnormal perfusion can reverse with treatment [6].
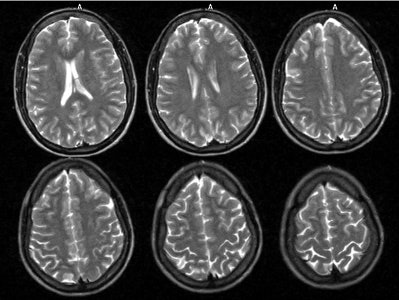
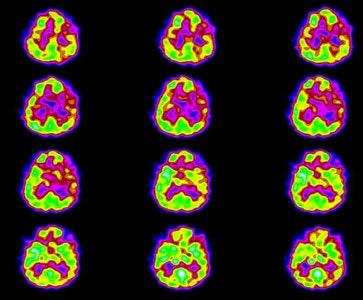
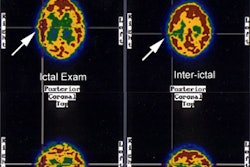
Neuro lyme: The patient below complained of intermittent right facial numbness, memory loss, and change in personality. Lyme titers were positive. MR imaging of the brain demonstrated no anatomic abnormality. SPECT imaging revealed multifocal areas of decreased perfusion in a pattern consistent with Lyme encephalopathy. |
|
REFERENCES:
(1) J Nucl Med 1991; Suess E, et al. Technetium-99m-d,1-hexamethylpropyleneamine oxime
(HMPAO) uptake and glutathione content in brain tumors. 32: 1675-81
(2) ANJR 1994, Aug,p.1369-73
(3) J Nucl Med 1998; Rieck H, et al. Discordance of Technetium-99m-HMPAO and Technetium-99m-ECD SPECT in herpes simplex encephalitis. 39: 1508-1510
(4) J Nucl Med 2001; Jager PL, et al. Radiolabeled amino acids: Basic aspects and clinical applications in oncology. 42: 432-445
(5) J Nucl Med 2001; Camargo EE. Brain SPECT in Neurology and Psychiatry. 42: 611-623
(6) Neurology 1997; Logigian EL, et al. Reversible cerebral hypoperfusion in Lyme encephalopathy. 49: 1661-1670
(7) J Nucl Med 1997; Sumiya H, et al. Brain perfusion SPECT in Lyme neuroborreliosis. 38: 1120-1122
(8) Clinical Infectious Diseases 1997; Fallon BA, et al. Functional brain imaging and neuropsychological testing in Lyme disease. 25 (Suppl 1): S57-63
(9) J Nucl Med 2009; Hattori N, et al. Differential SPECT activation patterns associated with PASAT performance may indicate frontocerebellar functional dissociation in chronic mild traumatic brain injury. 50: 1054-1061
(10) Radiology 2009; Agarwal R, Sze G. Neuro-lyme disease: MR imaging findings. 253: 167-173