Melanoma:
General:
Melanoma is the most aggressive form of skin cancer. The most
predictive factor for recurrence and prognosis is the tumor
thickness- measured in millimeters (Breslow thickness) [4]. For
localized lesions with a tumor thickness of less than 1.5 mm
(stage I or II), melanoma can be cured by total resection with a
5-yr overall survival approaching 90% [22] (early-stage melanoma
can be treated with surgery with a 5-year survival rate of 98.3%
[25]). However, in patients who develop metastases (stage IV) the
median survival rate is around 6-12 months and survival at 5 years
does not exceed 5% [22]. Between 6-10% of patients have detectable
metastases at the time of diagnosis [11]. Regional lymph nodes are
the most frequent site for metastases [1]. The presence of lymph
node metastases is also well established as a negative prognostic
indicator. Surgical excision of involved nodes is the most
effective treatment for either cure or local disease control [4].
Furthermore, 50-80% of patients with locoregional metastases and
nearly all patients with distant metastases will experience
disease recurrence after treatment [25]. Once distant metastases
occur, median survival is only about 4-6 months [16,19] and
therapy is always palliative [4].
Prognostic factors include Breslow thickness of the primary tumor, evidence of primary tumor ulceration, the mitotic rate of the primary tumor, the extent of local and regional lymph node involvement, and the presence of distant metastases [25].
FDG PET imaging is of limited use in patients with early stage
melanoma (I-II) disease because of their low risk for metastatic
disease [8,17]. Sentniel lymph node status is the single most
important prognostic factor for disease recurrence and survival
[25]. High risk for regional lymph node metastases include a
Brelow thickness ≥ 1.00 mm, primary tumor ulceration, high mitotic
index, vertical growth phase, male sex, young age, and high Clark
level of invasion [25]. These patients presently undergo surgical
resection of the lesion followed by examination of the sentinal
node [1]. The sentinal node is the first draining node of the
lymphatic bed draining the tumor- if the sentinal node is free of
tumor, the remainder of the nodes in that basin are likely to be
free of tumor. This approach obviates the need for a more radical
lymph node dissection [1]. Regional lymph node mets can be
categorized as micrometastases (clinically occult),
macrometastases, or in-transit or satellite mets [25]. Between
5-30% of patients with AJCC stage I-II melanoma are upstaged to
AJCC stage II following SLNB [25].
In patients with more advanced melanoma (thickness greater than
1-4mm, ulcerated lesions, or those with a high mitotic rate, or
AJCC stages III/IV) one may reasonable consider the utility of FDG
PET imaging for more effective tumor staging [16,19]. PET/CT
imaging seems to be more precise than PET imaging alone [19]. In a
meta-analysis, FDG-PET had a sensitivity of 83% and a specificity
of 85%, at initial staging [22].
Although FDG PET imaging has shown excellent results for the evaluation of cutaneous melanoma, the exam may lack sensitivity for the detection of uveal melanoma [14].
Presently, FDG PET is reimbursed for the following indications in patients with melanoma:
1- Evaluation of extranodal metastases at
initial staging or during follow-up after treatment
2- Evaluation for recurrent melanoma as an alternative to gallium scan
PET imaging is not reimbursed for the
evaluation of regional lymph node metastases for primary staging
Detection of Metastatic Disease:
Lymph node staging: For the evaluation of lymph node metastases FDG-PET has been suggested to have a sensitivity as high as 95%, a specificity of 84%, and an accuracy of 91% [2]. However, pooled data from multiple studies indicates that FDG PET has a very low sensitivity of only about 17% (0-40%) for the detection of sentinel lymph node metastases [16], particularly in patients with Stage I/II disease [25]. In general, the detection of nodal metastases is dependent upon the size of the metastasis [2,7,16]. Detection can be as high as 100% for metastases greater than 1 cm in size, a diameter of at least 6 mm is associated with a detection sensitivity of over 83%, while detection of metastases smaller than 5 mm is only 23% [2,7]. A tumor volume of greater than 78 mm3 is a associated with a sensitivity for detection of greater than 90% [7]. Because micrometastases to the sentinel node can be found in up to 38% of patients [13], FDG PET imaging should not replace sentinel node biopsy in the clinical management scheme of melanoma patients [5,16]. Overall, sentinel node biopsy is more sensitive than PET imaging for the detection of microscopic nodal metastases [8,11] with a reported sensitivity of 94%, and a specificity of 100% [4].Distant metastatic disease: With exception of MRI for staging brain and liver
metastatic disease, FDG PET/CT is superior to other imaging
modalities for the detection of distant metastatic disease [25].
Pooled data indicates that FDG-PET imaging is more accurate for
systemic staging, than for staging of regional lymph nodes [5].
Melanoma can spread widely and
unpredictably throughout the body [16]. FDG-PET can be useful in
detecting lymph node and distant metastases -- particularly in
stage III or IV high-risk patients (melanoma thickness greater
than 1.5 mm or Clark?s level IV) [1,3,6,17]. FDG-PET imaging has
been shown to be superior to conventional imaging for the
detection of melanoma metastases [3,11]. Studies have shown that
peripheral skin metastases as small as 3 mm can be detected [3].
Overall, pooled data for FDG-PET imaging in the detection of
melanoma metastases indicates a sensitivity of 79-83% (95%
confidence interval 66%-93%) and a specificity of 85-86% (95%
confidence interval 78%-95%) [5,7,19].
Impact on patient management/Prognosis:
PET scan results can have a significant impact on patient
management by detecting additional unsuspected sites of disease
(in one prospective study PET detected unexpected metastases in
12% of patients [21]) [9]. When PET is added to CT for the initial
staging of high-risk melanoma patients, management can be changed
in about 33% of patients (range 12% to 90% of cases) [9,15,19,21].
In one prospective study of patients with stage III disease, PET
findings resulted in a change in patient management in 15% of
cases [12]. In a separate prospective study of patients with
suspected recurrent melanoma, PET findings resulted in a change in
therapy in 40% of the cases [16]. Unfortunately, CT is still
superior to PET for the detection of lung metastases [11].
SUVmax, TLG and MTV are all predictors of disease free survival
[32].
|
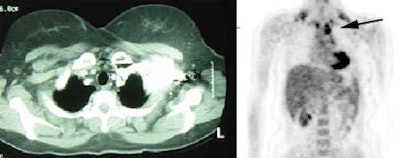
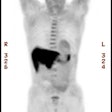
Metastatic melanoma: The patient shown below had a history of a lower extremity melanoma. CT imaging performed as part of the patient's evaluation revealed no evidence of metastatic disease. A FDG PET study demonstrated the presence of multiple lymph node metastases involving the supraclavicular and mediastinal nodes (black arrow). Beam hardening artifact from the I.V. contrast bolus obscured adequate evaluation of these regions on CT. PET imaging provides an excellent whole body survey and is a very sensitive exam for detecting melanoma metastases. Case courtesy of CTI PET Systems, Inc. |
|
|
|
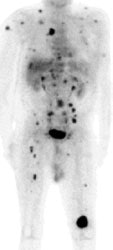
Widespread melanoma metastatses- PET alters patient management: The patient shown below was a 71 year old male with a history of melanoma on the left shoulder. A bone scan demonstrated the lesion in the left distal femur and also identified 4 spinal lesions. On CT, the abdomen had been interpreted as negative. The FDG PET exam revealed extensive metastatic disease throughout the body. The patient had been scheduled for tumor resection and total knee replacement surgery. After the PET exam the surgery was cancelled- avoiding both the cost and trauma of an ineffective operation. The exam was acquired on a Siemens ECAT EXACT PET scanner (manufactured by CTI). Case courtesy of Dr. Amjad Ali, M.D., Rush-Presbyterian-St. Luke's Medical Center, Chicago, IL and CTI PET Systems, Inc. |
|
|
|
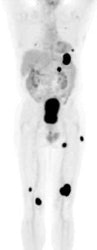
Widespread melanoma metastatses- The case below is from a 53 year old male with metastatic melanoma. The exam demonstrates multiple soft tissue, lung, and osseous metastases. Note the larger field of imaging for the evaluation of metastatic melanoma which extends to below the knees. Some centers will perform whole body examinations in patients with melanoma. Note the faint gonadal activity and the markedly amount of activity within the distended urinary bladder. The exam was performed on an ECAT EXACT PET scanner (CTI) using a dose of 10.2 mCi FDG. Case courtesy of Oregon Positron Imaging, LLC, Albany, Oregon and CTI (The power behind PET). Click image to view cine avi file (230K) |
|
|
Recurrent Melanoma:
Recurrent melanoma can occur anywhere in the body and the
patients initial stage determines the subsequent risk for disease
recurrence. Patients with stage I and II lesion are more likely to
have locoregional recurrences, while patients with stage III and
IV lesions more frequently present with distant metastases [10].
Patients with AJCC stage IIB disease have a 17% rate of recurrence
and patients with stage IIIA disease have a greater than 30%
recurrence rate, with most recurrences happening within the first
5 years [25].
FDG PET imaging can aid in the evaluation of patients with
suspected recurrent melanoma [10] and has been shown to be
superior to conventional imaging [17]. The sensitivity and
specificity of PET for detecting lesions on a per-patient basis
are 74-92% and 86-94%, respectively, compared to 57-81% and 45-87%
for conventional imaging [10,17]. PET is superior to CT in
detecting skin lesions, lymph node, abdominal, liver, and bone
metastases [10]. The overall accuracy of PET was 81%, compared to
52% for conventional imaging [10]. In another study, FDG PET was
found to be more accurate than conventional imaging in re-staging
and follow-up of melanoma with a site sensitivity of 92% versus
57.5%, and a specificity of 94% versus 45%, respectively [6]. The
addition of PET to conventional re-staging aids in the
identification of unsuspected sites of disease and treatment
planning can be changed in 20-36% of cases [9,10]. The net effect
of these changes can result in an overall cost savings (of about
$4,400 per patient) due to the prevention of unnecessary surgery
[9].
There is less data supporting the effectiveness and diagnostic
performance of FDG PET CT in detecting recurrence in asymptomatic
patients [25].
Treatment:
123I-BZA2 and 123I-BA52 are
melanin-targeted radiotracers for the identification of melanin
containing melanoma metastases [22,23]. Initial studies suggest
that radioimmunotherapy with 131I-BA52 may be of
benefit in patients with melanin containing melanoma metastases
[23].
Immune checkpoint inhibitors have demonstrated benefit for the
treatment of patients with advanced melanoma [29]. Agents include
cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) blocking
therapy (ipilmumab), programmed cell death protein 1 (PD-1)
blocking therapy (nivolumab, pembrolizumab), and programmed cell
death ligand 1 (PD-L1) blocking therapy (atezolizumab, avelumab,
durvalumab) [29].
Ipilimumab is a fully human monoclonal antibody (IgG1) that
promotes anti-tumor immunity by activating cytotoxic T lymphocytes
by blocking cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4),
an immune checkpoint molecule that down-regulates pathways of
T-cell activation [20,24]. The agent is effective in only 10-15%
of patients and is often associated with autoimmune adverse events
[24,25,26]. Note that patients that experience autoimmune adverse
events have been shown to be much more likely to respond to the
agent [26]. However, other authors report that the response rate
to combine nivolumab (PD-1) and ipilimumab (CTLA-4) treatment was
58% with a 5-year progression free survival of 36% [33].
Gastrointestinal and hepatic events typically occur after 6-7
weeks of therapy, and endocrine events occur later [24].
Programmed cell death ligand 1 (PD-L1) is part of an immune checkpoint system essential for preventing autoimmunity and cancer [28]. Tumor cells have developed the ability to co-opt these immune checkpoints to suppress anti-tumor immunity [28]. Advanced melanoma patients with over-expression of PD-L1 have been shown to have a 39% response rate to treatment with anti-PD-L1 therapy, compared to a 13% response rate in PD-L1 negative patients [28].
Autoimmune adverse events include:
- Enterocolitis (most common toxicity seen in 31-46% of
patients)- usually within the first 3 months of therapy and can be
segmental or diffuse
- Hypophysitis (5% of patients), hepatitis (3-9% of patients)
- Dermatitis, myopathy
- Pancreatitis
- Thyroiditis
- Nephritis
- Benign reactive lymphadenopathy in a sarcoid-like distribution
(5% of patients)
- Pneumonitis (5% of patients)
Monitoring treatment response to ipilimumab using radiologic
techniques is complex because lesions may initially enlarge and
tumor-regression responses are not often observed until after 3
months of treatment (pseudoprogression) [25,29]. Inflammatory
reactions and tumor progression often precede the treatment
response [25,29]. Patients with stable anatomic disease can
demonstrate modest to marked increased FDG uptake between 3-4
weeks following initiation of therapy likely related to an
inflammatory response to the therapy (related to tumor
infiltration by immune cells) and these patients tend to
demonstrate eventual tumor regression [29,30]. New lesions can
also be seen, but have been found to be associated with
progressive disease in only about half (55%) of cases [30]. In
patients with stable or decreasing target lesions, the appearance
of new lesions may be considered indeterminate [30]. The initial
expansion and recruitment of cytotoxic T lymphocytes after
checkpoint inhibition occur in lymph nodes systemically or in the
immediate draining nodal stations [34]. In the setting of
ipilimumab for melanoma, all patients with new mediastinal or
hilar nodal uptake on FDG PET/CT (sarcoid-like adenopathy)
ultimately demonstrated apparent clinical benefit from treatment
[34]. Short term followup in 1-2 months can be performed to assess
for change over time [25]. FDG PET scans can also demonstrate mild
tracer uptake in multiple lymph nodes after immune modulation
therapy [25].
Pseudoprogression is more commonly observed after treatment with
anti-CTLA-4 agents, as compared to anti-PD agents [34]. In
two-thirds of cases, pseudoprogression is observed before week 12
(early), whereas in one-third of cases it occurred after week 12
(delayed) [34].
Ocular melanoma:
Ocular melanoma is the most common noncutaneous melanoma and the
most common ocular primary in adults [27]. Ocular melanoma
represents about 4% of all cases of melanoma and 73% of cases of
noncutaneous melanoma [27]. Ocular melanoma has a predilection for
white patients (8-10x's higher rate compared to other races) and
the mean age at diagnosis is 60 years [27]. Sun exposure is
contested as a risk factor [27]. The lesion is often diagnosed
during routine eye exam [27]. Symptoms can include blurry vision,
photopsia (perceived flashes of light), or visual field defects
[27].
The choroid of the uveal tract is the most common site for ocular
melanoma and approximately 25% of choroidal melanomas are
amelanotic [27]. Uveal melanoma typically spreads hematogenously
because of lack of lymphatics in the uveal tract- most commonly to
the liver (90% of cases), lung (24% of cases), and bone (16% of
cases) [27]. Approximately half of patients with choroidal
melanoma will die of metastatic disease despite eradication of the
primary tumor and enucleation does not appear to confer a survival
benefit over occular sparing therapies [27]. The best predictor of
metastatic risk is genetic tumoral analysis for the presence of
monosomy 3 [27].
On CT, uveal melanoma generally appears as a non-specific
soft-tissue mass that is hyperdense relative to the vitreous humor
and shows mild-to-moderate enhancement with contrast [27].
REFERENCES:
(1) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
(2) J Nucl Med 2000; Crippa F, et al. Which kinds of lymph node metastases can FDG PET detect? A clinical study in melanoma. 41: 1491-1494
(3) Cancer 1998; Rinne D, et al. Primary staging and follow-up of high risk melanoma patients with whole-body 18-F-fluorodeoxyglucose positron emission tomography: Results of a prospective study of 100 patients. 82: 1664-71
(4) J Cancer Res Clin Oncol 2000; Ilknur AK, et al. Positron emission tomography with 2-[18F] fluoro-2-deoxy-D-glucose in oncology: Part II: The clinical value in detecting and staging primary tumours. 126: 560-574
(5) Cancer 2001; Mijnhout GS, et al. Systematic review of the diagnostic accuracy of 18F-Fluorodeoxyglucose positron emission tomography in melanoma patients. 91: 1530-42
(6) Radiographics 2003; Kostakoglu L, et al. Clinical role of FDG PET in evaluation of cancer patients. 23: 315-340
(7) J Nucl Med 2003; Cobben DCP, et al. 3'-18F-fluoro-3'-deoxy-L-thymidine: a new tracer for staging metastatic melanoma? 44: 1927-1932
(8) J Nucl Med 2004; Schoder H, et al. PET/CT in oncology: integration into clinical management of lymphoma, melanoma, and gastrointestinal malignancies. 45 (Suppl): 72S-81S
(9) Radiology 2004; Rohren EM, et al. Clinical applications of PET in oncology. 231: 305-332
(10) J Nucl Med 2004; Fuster D, et al. Is 18F-FDG PET more accurate than standard diagnostic procedures in the detection of suspected recurrent melanoma? 45: 1323-1327
(11) Radiol Clin N Am 2005; Kumar R, et al. Fluorodeoxyglucose-PET in the management of malignant melanoma. 43: 23-33
(12) Cancer 2000; Tyler DS, et al. Positron emission tomography scanning in malignant melanoma. 89: 1019-1025
(13) J Nucl Med 2006; Rossi CR, et al. The impact of lymphoscintigraphy technique on the outcome of sentinel node biopsy in 1,313 patients with cutaneous melanoma: an Italian mutlicentric study (SOLISM-IMI). 47: 234-241
(14) J Nucl Med 2006; Kato K, et al. Low efficacy of 18F-FDG PET for detection of uveal malignant melanoma compared with 123I-IMP SPECT. 47: 404-409
(15) Ann Surg Oncol 2006; Brady MS, et al. Utility of preoperative [(18)]f fluorodeoxyglucose-positron emission tomography scanning in high-risk melanoma patients. 13: 525-32
(16) J Nucl Med 2006; Belhocine TZ, et al. Role of nuclear medicine in the management of cutaneous malignant melanoma. 47: 957-967
(17) Radiology 2007; Blodgett TM, et al. PET/CT: form and function. 242: 360-38
(18) Radiology 2007; Strobel K, et al. High-risk melanoma: accuracy of FDG PET/CT with added CT morphologic information for detection of metastases. 244: 566-574
(19) Radiology 2008; Krug B, et al. Role of PET in the initial
staging of cutaneous malignant melanoma: a systematic review. 249:
836-844
(20) AJR 2012; Nishino M, et al. Personalized tumor response
assessment in the era of molecular medicine: cancer-specific and
therapy-specific response criteria to complement pitfalls of
RECIST. 198: 737-745
(21) AJR 2012; Bronstein Y, et al. PET/CT in the management of
patients with stage IIIC and IV metastatic melanoma considered
candidates for surgery: evaluation of the additive value after
conventional imaging. 198: 902-908
(22) J Nucl Med 2014; Cachin F, et al. 123I-BZA2 as a
melanin-targeted radiotracer for the identification of melanoma
metastases: results and perspectives of a multicenter phase III
clinical trial. 55: 15-22
(23) J Nucl Med 2014; Mier W, et al. Radiopharmaceutical therapy
of patients with metastasized melanoma with the melanin-binding
benzamide 131I-BA52. 55: 9-14
(24) AJR 2015; Howard SA, et al Beyond the vascular endothelial
growth factor axis: update on the role of imaging in
nonantiangiogenic molecular targeted therapy. 204: 919-932
(25) AJR 2015; Perng P, et al. 18F-FDG PET/CT and
melanoma: staging, immune modulation and mutation-targeted therapy
assessment, and prognosis. 205: 259-270
(26) AJR 2016; Holler Howard SA, et al. A new look at toxicity in
the era of precision oncology: relationship with tumor response,
and effect on metastasectomy. 207: 4-14
(27) AJR 2017; Wong VK, et al. Clinical and imaging features of
noncutaneous melanoma. 208: 942-959
(28) J Nucl Med 2017; Nedrow JR, et al. Imaging of programmed
cell death ligand 1: impact of protein concentration on
distribution of anti-PD-L1 SPECT agents in an immunocompetant
murine model. 58: 1560-1566
(29) J Nucl Med 2017; Cho SY, et al. Prediction of response to
immune checkpoint inhibitor therapy using early-time-point 18F-FDG
PET/CT imaging in patients with advanced melanoma. 58: 1421-1428
(30) J Nucl Med 2019; Ito K, et al. 18F-FDG PET/CT
for monitoring of ipilimumab therapy in patients with metastatic
melanoma. 60: 335-341
(31) AJR 2019; Sanli Y, et al. Tumor heterogeneity on FDG PET/CT
and immunotherapy: an imaging biomarker for predicting treatment
response in patients with metastatic melanoma. 212: 1318-1326
(32) Clin Nucl Med 2016; Son SH, et al. Prognostic value of
volumetric parameters measured by pretreatment 18F-FDG
PE/CT in patients with cutaneous melanoma. 41: e266-273
(33) J Nucl Med 2020; Niemeiher AL, et al. Imaging responses to
immunotherapy with novel PET tracers. 61: 641-642
(34) J Nucl Med 2020; Iravani A, Hicks RJ. Imaging the cancer immune environment and its response to pharnacologic intervention, part 1: the role of 18F-FDG PET/CT. 61: 943-950