If anyone needs reliable computer-aided detection, it's radiologists who screen for lung cancer. They'll tell you that reading chest CTs is tedious and time-consuming work, and that doing it correctly means scrolling in and out of each lung quadrant in search of suspicious nodules.
Follow-up presents additional challenges. More than half of the patients in the core screening group -- aging smokers and ex-smokers -- present with at least one nodule at the first exam. Biopsying each one would be expensive and risky. So radiologists must typically wait and watch for evidence of nodules that have grown or changed significantly by the time of follow-up, hoping all the while that some nascent lesion doesn't begin to grow aggressively and become inoperable before the next exam. It's a lot of surveillance.
Then there are the patient numbers, potentially so large as to be awe-inspiring. Fully half of the U.S. adult population consists of smokers or former smokers. One of every 18 men and one of every 12 women in the U.S. will eventually be diagnosed with lung cancer, which kills more people each year than breast, prostate, and colon cancer combined.
There is little hope of a magic bullet. However, radiologists got a good shot of encouragement at the 2002 RSNA meeting in Chicago, where several groups presented their latest efforts to develop reliable lung CAD schemes. Initial results with four of the systems are presented here.
Move over, Big Blue
A group from Stanford University in Stanford, CA, pitted man against machine in a study designed to compare double reading of chest scans by comparing CAD versus rad. By some measures, the machine won.
"Double reading has been shown to improve sensitivity in chest x-ray and mammography...(and) may offer the benefits of double reading without requiring a second radiologist," said Dr. John Lyo.
In an effort to determine whether CAD can improve on radiologists' readings, the group compared the performance of its CAD technique to that of a radiologist, then compared the results of double reading by two radiologists to that of a single radiologist plus CAD for the detection of pulmonary nodules 3 mm and larger.
The team recruited three fellowship-trained chest or body radiologists, each of whom reviewed 21 scans individually and dictated all found nodules 3 mm and larger. The nodules were graded on a five-point scale, 5 being the most definite.
The reference standard regarding the actual number of nodules was established by consensus of two highly experienced thoracic radiologists, who read each dataset twice and incorporated CAD results as well. This process detected a total of 100 nodules 3 mm or larger in 14 patients, with no nodules in the remaining 7 patients. (In pairings of one radiologist plus CAD or two radiologists, a positive result by one reader was considered positive for the double reading.)
Working without CAD, the three radiologists achieved sensitivities ranging from 40%-62% for nodules 3 mm and larger, with about 1-3 false-positives per scan, Lyo said.
Then CAD alone took a crack at the same 21 cases, achieving sensitivity as high as 95% when a second-pass false-positive filter was allowed to produce as many as 50 false positives. When the filter was set to limit false positives to a level comparable to that of the radiologists (2-6 false positives per case), CAD's sensitivity was "inferior to the radiologists but in the ballpark," Lyo said.
(In practice, the optimal setting for the second-pass filter may boil down to the radiologist's preference. Some may prefer to look at as many potential nodules as possible and rule them out manually, while others might want the machine to filter out the least likely nodule candidates for them.)
For 6-10 mm nodules only, CAD achieved 91% sensitivity at under 2 false positives per scan -- comparable to radiologists' results -- with a maximum of 96% sensitivity and 36 false positives when the filter was set to allow unlimited false-positives.
The radiologists spent a mean of 7.5 minutes on each case, "but we found that the amount of time spent didn't necessarily correlate with their maximum sensitivity, so we decided to test the three readers and CAD in every possible combination," Lyo said. This model assumed that the radiologists would verify their reading by looking at the results of either CAD or the second radiologist.
Adding a second reader increased sensitivity by about 13% over that of a single reader. But a single reader plus CAD increased sensitivity by 22% when the CAD was allowed to have up to five false positives per scan.
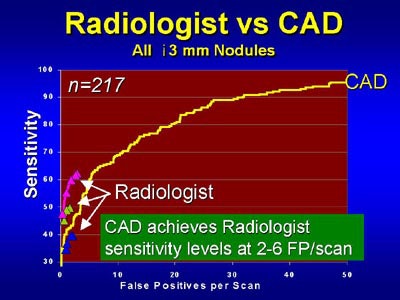 |
| Above, CAD's sensitivity for the detection of lung nodules is comparable to that of radiologists at similar false-positive rates, and exceeds radiologists' sensitivity when additional false-positives are permitted in CAD. Chart courtesy of Dr. John Lyo |
"If you allow CAD to have up to 30 false positives per scan, you could increase the (radiologist plus CAD) sensitivity over (that of a single) reader by about 43%," Lyo said. For nodules 6 mm and larger, however, one reader plus CAD achieved about the same results as two readers.
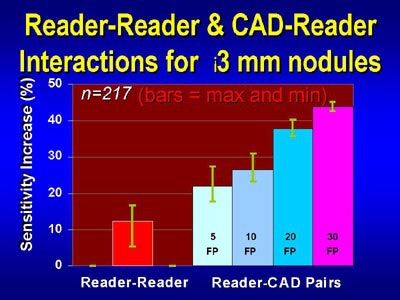 |
| For the detection of pulmonary nodules 3 mm and larger, pairing CAD with a reader yielded higher sensitivity than that of two readers working together -- at several levels of permitted false-positive results. Chart courtesy of Dr. John Lyo. |
"In summary, CAD has a higher overall sensitivity than radiologists, especially for small nodules," Lyo said. "CAD appears to improve sensitivity for the detection of pulmonary nodules, and may improve it above radiologist/radiologist total reading."
Lyo said the group is currently assessing how CAD affects interpretation times.
Automated follow-up of lung nodules
The next presentation addressed the herculean task of following up lung nodules detected in initial screening. Carol Novak, Ph.D. from Siemens Research in Princeton, NJ, discussed a study performed in cooperation with thoracic radiologist Dr. David Naidich at the New York University Medical Center in New York City.
"The problem we're addressing affects every chest radiologist in the room," Novak said. "Half of the patients who come in for screening have nodules that require some sort of follow-up -- not large enough to justify biopsy, but too large to be ignored. In fact, chest radiologists have complained that (nodule follow-up) is one of the tasks they dread the most."
To confront the task of matching up vast quantities of nodules to see if they have grown by the time of follow-up, radiologists need an automated system to match nodules from initial studies to those of follow-up exams, Novak said.
The burden can be expected to grow as radiologists find more and more nodules using multidetector-row CT to screen for lung cancer. Even the fact that CAD systems are finding more nodules adds to the follow-up workload, Novak said.
Siemens' prototype system, called Real-Time Automatic Matching or RAM, does not find the nodules in the initial exam data, a job that was accomplished in the study by another Siemens lung CAD application, and alternatively by means of traditional interpretation by radiologists.
Rather, the RAM system coregisters images from two scans, the initial scan and the follow-up scan, by means of a two-step process. First the computer performs a rough alignment of the lungs by identifying the lung area on each axial slice, and computing the best fit between the lung areas.
The user then scrolls through the study, finds a nodule, selects a point, and asks the system to find the corresponding nodule in the other study. By analyzing adjacent anatomic structures, the system then generates a more precise location "that will hopefully be right on the nodule," Novak said.
Once all of the nodules in the first exam have been matched to the nodules in the follow-up exam, the radiologist can determine whether any have grown or changed significantly since the first exam, and therefore warrant further examination.
The group tested the system by performing thoracic CT twice in each of 19 patients, an initial scan with follow-up (mean 12 months, range 3-18 months) later. A mixture of low-dose and standard-dose thoracic studies were acquired with a four-slice scanner, generating a mean of 283 slices per scan, according to Novak.
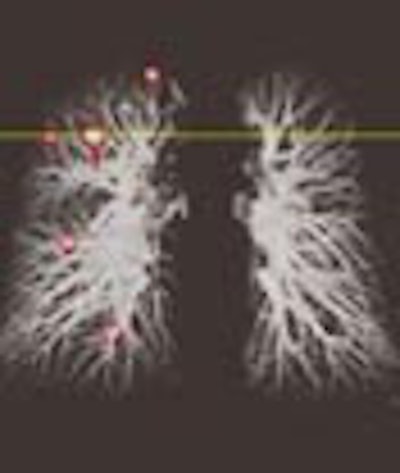
In all, 240 nodules or nodule-like locations were found. The mean Euclidian distance was only 4.07 mm between the nodules' location as determined manually, and that predicted by the RAM application, Novak said. The mean distance was 8.9 mm for the global registration method used to roughly align the two studies at startup (p<0.001).
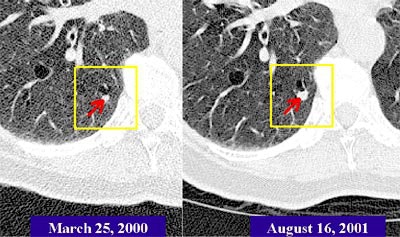 |
| The Real-Time Automatic Matching System (RAM) is designed to automatically determine the corresponding location of nodules in another CT lung study. In the patient above, RAM located a nodule that had grown significantly over 17 months, even though radiation dose and other acquisition parameters had changed. Images courtesy of Siemens Corporate Research. |
"We see that (63.5%) of the (RAM) matches were exactly on the structure, and more than 90% were within 5 mm," she said. Computation time is very reasonable, she said; the computer performs the rough alignment of the entire study in about 15 seconds. Initial and follow-up scans of the same patient demonstrate the robustness of the system.
"Despite the fact that the nodule is much smaller in the previous study, despite the fact there is a quality difference between the two scans, RAM still managed to find it," Novak said. "This is a very reasonable system to use in the clinical setting because the computation time is so reasonable."
Session moderator Dr. Ella Kazerooni from the University of Michigan asked Novak if there were any discernable patterns or features in cases where the exams were not closely matched.
In the current application, matching is less accurate when the patient is rotated significantly on the table, or sitting up during one exam but not the other, Novak responded. She added that although the system can accommodate a range of exposure levels, the studies being compared should contain the same approximate number of slices, and be acquired on scanners with similar detector configurations.
3-D morphologic matching algorithms
Multidetector-row CT has led to dramatic increases in the number of images radiologists must evaluate in lung cancer screening, according to Dr. K. Ty Bae from Washington University in St. Louis.
A fast and reliable CAD system could help radiologists deal with the resulting "nodule nightmare," Bae said in his presentation at the RSNA show. Yet most 2-D CAD schemes have had only limited success in detecting nodules, Bae said, so his group decided to develop and test a 3-D morphologic matching application. His group's program uses 3-D volumetric data from high-resolution multislice CT to classify nodules more accurately than existing 2-D techniques.
The team's Matlab-based application works by first segmenting the parenchyma, nodules, and vessels of the lung region. It then extracts and labels nodule candidates, which are evaluated for shape features including volume, size, compactness, and elongation. A process that includes 3-D region-growing and morphologic matching is then applied iteratively in order to classify the candidates into isolated nodules, pleural nodules, juxtavascular nodules, or non-nodules, which are treated separately by the application, Bae said.
"The isolated and pleural nodules are detected by applying a rule-based system based on their geometric and shape features," Bae said. "For juxtavascular nodules we have to go through another step, multilevel matching. First we disassociate the nodule from the vessel, then we can subsequently apply the same kind of rule to detect the nodule."
In the study, 20 consecutive patients underwent chest CT imaging using 4 x 1-mm collimation, 0.5-second gantry rotation, 120 kVp, 120 mAs, 1-mm slice thickness, and 1-mm reconstruction interval.
The reference standard was determined by an experienced chest radiologist who read the images twice, without and with the aid of the Matlab CAD software. This method resulted in the detection of 164 nodules (mean 5.4 mm diameter), including 27 nodules 10 mm or greater, 80 nodules 5-10 mm in diameter, and 57 nodules 3-5 mm in diameter.
Compared to the reference standard, CAD alone was 95.1% sensitive (156/164) for the detection of all nodules, delivering 92.6% sensitivity for nodules 10 mm and larger, 98.8% sensitivity for nodules 5-10 mm, and 91.2% sensitivity for nodules 3-5 mm in diameter, with 138 false positives (0.84 false positives per true-positive nodule).
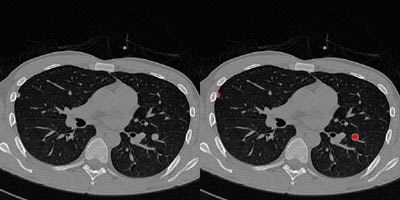 |
| Above right, a pleural nodule in the right lung and a juxtavascular nodule in the left lung are accurately detected by the 3-D CAD program. No false-negative or false-positive nodules were observed in this case. Images courtesy of Dr. K. Ty Bae. |
"Our 3-D based CAD program can detect nodules including small, pleural and juxtavascular nodules in CT images with high sensitivity and a low false-positive rate," Bae concluded.
Processing time is slow, about 20 minutes per case, Bae said. But he added that the software could easily be optimized to work in less than a couple of minutes. Another limitation is that the current shape-based analysis algorithm does not recognize textural features such as ground-glass nodules, Bae said.
CAD improves results for less experienced readers
Finally, Dr. Joseph Schoepf from Massachusetts General Hospital in Boston concluded that the accuracy of radiologists could be improved significantly when CAD was added to the evaluation of high-resolution multislice CT lung scans.
In the study, two readers independently evaluated 22 CT datasets, once without CAD, and once again two weeks later with the aid of CAD (ImageChecker, R2 Technology, Sunnyvale, CA). Reader 1 had one year of experience; Reader 2 had six years of experience.
The reference standard was determined by calculating all nodules detected by the two radiologists plus CAD plus an adjudicator, and nodules were rated based on high, medium, or low "actionability." (Actionability refers to the need to take action such as follow-up or biopsy based on the nodule's size and probability of malignancy.)
All readers together with the aid of CAD detected a total of 107 nodules of high and medium actionability. Without CAD, Reader 1 and Reader 2 achieved 78.8% and 93.3% sensitivity, respectively, for the detection of high and medium lung nodules compared to the reference standard.
With CAD, Reader 1 and Reader 2 achieved sensitivity of 86.5% and 95.5%, respectively, compared with the reference standard. In addition, reading times were reduced by as much as 42%, Schoepf said.
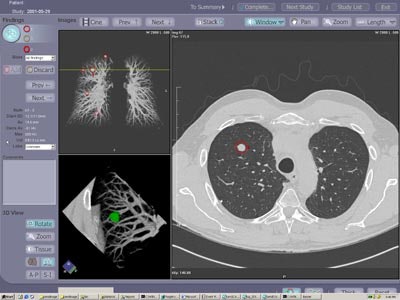 |
| The investigational ImageChecker CT CAD software is designed for the early detection of clinically significant lung nodules in CT. The CAD algorithm highlights potential areas of interest and provides measurement and characterization information on detected lesions. Image courtesy of R2 Technology. |
CAD improves both reading times and sensitivity for all readers, Schoepf said. Moreover, CAD improved the results of less-experienced readers to the point where they were comparable to those of far more experienced readers, he concluded.
AuntMinnie.com staff writer
February 13, 2003
Related Reading
Lung cancer screening not cost-effective, say Johns Hopkins researchers, January 14, 2003
Self-referred screening patients are far less likely to find abnormal results, January 3, 2003
Worrisome radiation dose seen in CT lung screening, follow-up, December 23, 2002
Researchers compare CAD systems for mammography, December 6, 2002
Copyright © 2003 AuntMinnie.com