Rewriting a page from standard clinical practice, CAD developers are using automated matching of prone and supine virtual colonoscopy datasets as their primary method of reducing false-positive candidates. And the nascent technique worked reasonably well, so long as the data were relatively free of collapsed segments and residual fecal matter.
"At present, CAD schemes analyze the supine and prone CTC (VC) datasets of a patient independently, as if they represented different colons," wrote Dr. Janne Näppi and colleagues from the University of Chicago in the June Academic Radiology. "Conversely, radiologists can visualize both datasets of a patient simultaneously for additional diagnostic evidence. For example, a true polyp tends to stay in the same region in the supine and prone positions, whereas mobile stool may move freely around the colon and can be found in different supine and prone locations" (Academic Radiology, June 2005, Vol. 12, pp. 695-707).
Detecting such movement requires correspondence between prone and supine datasets, however, and the severe displacement of the colon that may occur when the patient changes positions makes this a difficult job for CAD. And as many as a quarter of the polyp candidates on the group's previous CAD scheme were associated with stool, the team noted, and even though CAD analyzes the internal structure of the finding, some stool mimics the internal structure of polyps.
Previous attempts at coregistration were based on colon centerlines, but the authors believe their CAD approach, based on a series of region-growing and distance-transform steps, can potentially do a better job of eliminating more false-positive candidates without inadvertently eliminating true polyps that may not be visible in one dataset or the other.
In their study, Näppi and Dr. Akihiko Okamura; Hans Frimmel, Ph.D.; Dr. Abraham Dachman; and Hiro Yoshida, Ph.D., retrospectively examined 121 VC datasets using their CAD algorithm. The patients had undergone cathartic cleansing prior to same-day virtual and conventional colonoscopy.
"In this study, we developed a new method for reducing FP (false-positive) polyp candidates based on correspondence between the supine and prone datasets," they wrote.
The method consists of the following four steps:
- Detection of anatomic landmarks
- Supine-prone registration by a distance transform
- Establishment of the supine-prone correspondence (SPC) of colonic segments
- Reduction of polyp candidates based on the SPC
The CT images used to test the CAD system were acquired on HiSpeed CTi and LightSpeed QX/i scanners (GE Healthcare, Chalfont St. Giles, U.K.) with collimation of 2.5-5 mm, slice thickness of 1.5-5 mm, reconstruction intervals of 1-5 mm, tube currents of 50-200 mAs, and 120 kVp.
"All cases were of diagnostic quality, but could contain feces, fluid, poorly distended colons, and motion artifacts," the team wrote.
SPC CAD scheme
The method identified six landmarks from each case, including the cecum, hepatic flexure, splenic flexure, sigmoid junction, rectosigmoid junction, and anus. The authors expected colon mobility to be smallest in these regions.
"The cecum ... is detected by use of downward region-growing from the hepatic flexure," they wrote, explaining one of the steps in SPC.
"During the downward region-growing at voxel p, the axial coordinates of the neighboring voxels of p added to the growing region may not be higher than p. Because the region-growing may spread to the transverse colon, we need to identify the branch representing the ascending colon in the grown region. This is done by first locating the voxel pB, which has the lowest axial coordinate in the grown region, and then locating the left-most grown voxel, pL, between the axial locations of pB and hepatic flexure. The cecum is detected as the voxel with the lowest axial coordinate in the grown region below pL."
As the region-growing steps were applied for each of the six landmarks, the voxels added to each grown region had to have a value of less than -970 HU to ensure that the colonic lumen between landmarks was not poorly distended or collapsed.
Particularly in the presence of poorly distended segments, "the locations of the automatically established landmarks might not match precisely with expected colon anatomy," the team wrote. "However, in our application it suffices that the established locations match in the supine and prone datasets. A number of checks ... are performed to ensure that the established landmarks are suitable for determining an SPC." The steps are described briefly below:
Supine-prone registration by distance transform: Once the landmarks have been established, a breadth-first region-growing process is applied across the colon from the first valid landmark, and the lumen distance of each grown voxel is calculated. Then, to resolve the ambiguous distance values, a second breadth-first region-growing process is applied from the last landmark covered by each region-growing. A third region-growing process is applied if the region-growing failed to reach a valid landmark, and the supine and prone lumens are registered by linear scaling of the distance values in the prone dataset, the authors wrote.
Colonic segments and quality assessment: After point-based lumen registration, a similar distance value in the supine and prone lumens indicates a similar location of the colon. At this point the correspondence is only approximate, however, so a region-based correspondence is applied by dividing the colonic lumen into overlapping segments that have a match in the prone and supine datasets.
"The final segments are extended symmetrically so that the overlap between adjacent segments is equal to the distance specified by the overlap parameter," the authors wrote.
Each segment is checked for adequacy of distension and preparation, ensuring that no true-positive detections are eliminated. Polyp candidates seen in both datasets are presumed valid. If a polyp is found in only one dataset, it is presumed invalid unless the segment is poorly distended, or is covered by stool or retained fluid that would cover the matching polyp in the other dataset.
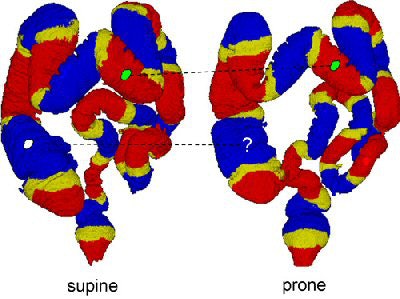
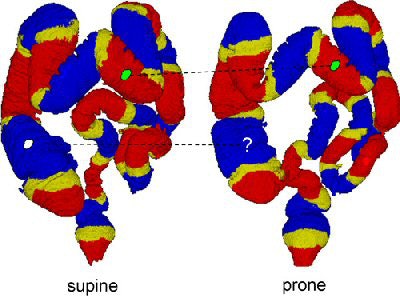 |
| The region-based correspondence between supine (left) and prone (right) datasets of a patient is established by dividing the colon into overlapping regions. Yellow regions indicate overlap between the two neighboring regions that are colored red or blue. If both of the corresponding regions in supine and prone datasets contain a polyp candidate (irregular green patches connected by dotted line), it is kept as a detected polyp. If only one of the corresponding regions contains a polyp candidate (irregular white patch), it may be removed as a false-positive detection. Image courtesy of Dr. Hiro Yoshida of Massachusetts General Hospital and Harvard Medical School in Boston. |
"Because we use cases of patients with cleansed colons, the solid stool ... is (generally) not large enough to cover polyps," the team wrote. "Furthermore, the solid stool tends to be detected as polyp candidates because of the polyp-like shape characteristics."
The CAD doesn't generally detect fluid-submerged polyps, however, and for this reason the fluid is detected by scanning the coronal cut-planes for flat surfaces. If any are found, the segment is eliminated from consideration, again ensuring that true-positive polyp candidates are not excluded.
Reducing false positives
During the previously described polyp detection process, "a thick region encompassing the colonic wall is extracted by use of an automated method (in this case, the centerline-based colon segmentation), and polypoid shapes are detected from the extracted region by use of hysteresis thresholding based on the shape index and curvedness features," according to Näppi et al.
All polyp candidates that appear in one dataset only are eliminated as potential false positives, but only if SPC is established. Mobile stool in one dataset is eliminated by means of the SPC method if it moves to a new segment in the other dataset and there are no other polyp candidates in the segments. Incidental detections that are seen in only one dataset are also eliminated. Then, after the SPC method is applied, the remaining polyp candidates are used as input to a Bayesian neural network that further reduces false positives based on shape and texture features, producing the final CAD output.
Four experiments were undertaken to evaluate the performance of the SPC method, including three that measured the accuracy of the computation steps in the region-based correspondence. A gastroenterologist experienced in VC interpretation evaluated the 2D and 3D virtual colonoscopy datasets. Finally, the team measured the SPC's effectiveness in reducing false-positive candidates.
Results
"Complete or partial correspondence was established in 71% of the cases," Näppi and colleagues wrote. "Based on a leave-one-patient-out evaluation, application of the method reduced 19% of false-positive results reported by our CAD scheme at a 90.5% by-polyp detection sensitivity, without loss of any true-positive results. The resulting CAD scheme yielded 2.4 false-positive results per patient without the use of the method."
The technique, developed specifically for reducing false positives in VC CAD, was the first to address the automated reduction of false positives using supine-prone correspondence, according to the authors. It safeguards against the inadvertent elimination of true positives, and has the further advantage of controlling uncertainties related to supine-prone registration and the perceived movement of polyps. A polyp-matching approach, on the other hand, would be rendered inadequate by the difficulty of establishing supine-prone correspondence in every case, by the tortuous morphology of colon, and by the frequency of poorly distended segments (only 7% of the colons were deemed fully distended).
"Misplacement of the established landmarks generally was caused by invisible colonic lumen at the anatomically correct location of a landmark," with the sigmoid junction proving to be the most unreliable as it was frequently collapsed, according to the researchers.
They added that the study should be validated in a larger cohort, and that the use of fluid and fecal tagging should be explored to increase accuracy by enabling the analysis of the fecal- or fluid-filled segments with the SPC method.
Nevertheless, the method yielded a "19% reduction of false-positive results at a 90.5% by-polyp detection sensitivity level without loss of any true-positive results," the team noted. "The results indicate that the method is potentially useful in improving the specificity of CAD in (VC)."
By Eric Barnes
AuntMinnie.com staff writer
June 28, 2005
Related Reading
3D topological thinning improves colon centerline tracking in VC, June 8, 2005
VC CAD shows progress toward clinical use, June 3, 2005
CAD struggles through tagged, subtracted VC data, May 18, 2005
Support vector machines boost accuracy of VC CAD, May 3, 2005
New VC CAD applications show promise as second reader, March 4, 2005
Low-prep VC study finds CAD can be fooled, December 23, 2004
Copyright © 2005 AuntMinnie.com