It was one of the bigger RSNA marketing coups in recent memory: With the radiology community expecting the commercial launch of a 256-detector-row CT scanner, Toshiba America Medical Systems switched gears and instead served up Aquilion One, a 320-element scanner that caught many RSNA attendees by surprise.
But the launch of Aquilion One raised as many questions as it answered. Is it clinically necessary to have a CT scanner with 320 detector rows when 256 slices represents a fourfold increase over the state of the art? How would the Tustin, CA, company address the many issues that complicate multislice CT, such as capital acquisition costs, data overload, and radiation dose? And how had the company managed to keep its launch such a closely guarded secret?
Beyond 64-slice
 |
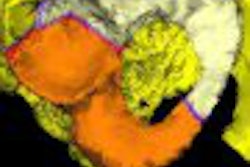
| Aquilion One stroke protocol shows pure arterial and venous anatomy displayed as a single fused volume. All images courtesy of Toshiba America Medical Systems. |
The origins of Aquilion One derived from Toshiba's plans to develop a successor to its Aquilion 64 system, first introduced in June 2004, according to Doug Ryan, senior director of the company's CT business unit. While multislice CT development up to that point had concentrated on producing more detector rows with higher temporal resolution for cardiac studies, Toshiba settled on an approach that aimed to cover an entire organ in a single rotation of the scanner's gantry.
This approach confers a number of advantages, the company believes. First and foremost, it eliminates the need to reconstruct images from multiple gantry passes, which both reduces the higher radiation dose caused by overlapping slices and also reduces reconstruction artifacts.
The firm first disclosed its work on 256-slice CT as a work-in-progress at the RSNA show in December 2004. The scanner was based on Aquilion 64 architecture, with a 0.5-mm slice width and 256 detector rows, producing 12.8 cm of coverage per gantry rotation.
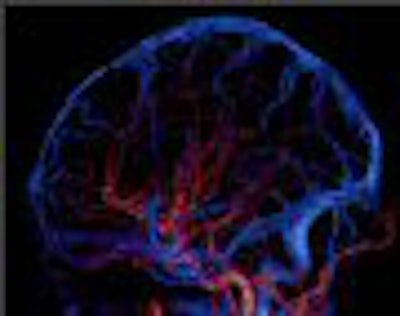
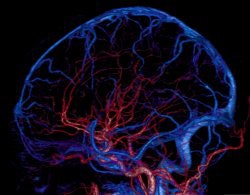
Two prototype systems were installed in 2007, one at Johns Hopkins University in Baltimore and the other at Fujita University in Tokyo. These early users for the most part presented positive results with the system, in particular demonstrating its utility in capturing whole-organ brain and cardiac images from human patients.
At the same time, Toshiba's clinical partners were telling the company that 12.8 cm of coverage might not actually be enough to accomplish the goal of whole-organ scanning in a single rotation, according to Ryan.
"We know from brain work that the ability to cover more than 12.8 cm in the brain was important in doing whole-brain perfusion," Ryan said. "In cardiac imaging on 256, on a majority of patients it was fine, but on 30% of cases we were clipping data."
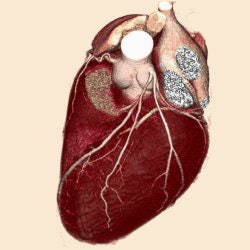 |
| A 3D volume heart acquired with Aquilion One within one heartbeat. |
Once the decision was made to make the next-generation commercial product a 320-detector-row system, it was technically a short leap to scale the system up by 25%, according to Ryan. The company's commercialization strategy was more complicated, however.
A stealth campaign
Toshiba decided that since its main goal was to promote the concept of dynamic volume CT of whole organs, whether it was accomplished with a 256- or 320-row architecture was irrelevant, Ryan said. So the company continued its campaign of discussing the clinical benefits of the whole-organ approach using 256-row CT, while secretly laying the groundwork for a better machine.
It wasn't as easy as it sounds -- radiology is a notoriously gossipy industry, so Toshiba had a number of its clinical and business partners who were working on the 320-detector-row system sign nondisclosure agreements (NDAs). That included executives at Vital Images, the Minnetonka, MN, firm that develops the advanced visualization software used to power Aquilion consoles.
Toshiba also requested NDAs from its clinical partners once it began installing Aquilion One at clinical sites in October 2007. The scanner went into five sites at that time: Fujita University in Japan, Johns Hopkins University in Baltimore, Brigham & Women's Hospital in Boston, the University of Toronto in Canada, and Charité Hospital in Berlin.
Toshiba even kept the 320-detector-row system a secret from much of its staff in the U.S. Employees were planning for the launch at the 2007 RSNA show of a system they knew would be called Aquilion One, but most believed that the unit would be a 256-slice system, Ryan said. Perhaps only 10 Toshiba employees outside of Japan actually knew that AquilionOne would be a 320-row system -- the rest learned just a couple days before the RSNA show began, he said.
Finally, Toshiba secured U.S. Food and Drug Administration 510(k) clearance for Aquilion One in October 2007. The scanner had already been cleared as a 256-slice model in October 2006, but the company chose to secure an additional clearance for the new configuration.
Remaining questions
As Toshiba prepares for the scanner's commercial rollout in summer 2008, the company has taken care to address each of the potential arguments against the system. With respect to data overload, the company is working with Vital Images and another partner, PACS firm McKesson of Burnaby, British Columbia, on developing software that will address the complications involved in data transfer and image interpretation of larger datasets.
With respect to radiation dose, the company points out that eliminating the overlapping slices inherent to helical CT produces an immediate benefit in reducing dose. Ultimately, the radiation dose level depends on the type of acquisition protocol being used, regardless of whether it's 320-, 64-, or 16-slice CT, according to the company.
One thing the company can't dance around is the price tag of the system, estimated in the $2.5 million range. Toshiba acknowledges that buying an AquilionONE will be costly, but says the scanner will produce overall cost savings to the healthcare system by reducing expenses for diagnosing and treating certain disease states. For example, AquilionOne will be able to conduct stroke assessments in 10 minutes that might otherwise have required both CT and MRI over a time period of several hours.
"The technology is very expensive -- this is one of the most technology-enabled devices today," Ryan said. "In talking to clinical partners, they urged us to bring this to market. They think the cost savings in healthcare will be demonstrated. It remains to Toshiba to prove that."
By Brian Casey
AuntMinnie.com staff writer
December 24, 2007
Related Reading
Toshiba, TomTec to partner on cardiac 4D ultrasound, December 6, 2007
Toshiba adds to its education initiative, December 3, 2007
Vital Images, Toshiba ink distribution deal, November 27, 2007
Aquilion One 320-slice CT scanner spearheads Toshiba RSNA launches, November 26, 2007
Toshiba launches contrast-free MRI technique, November 15, 2007
Copyright © 2007 AuntMinnie.com