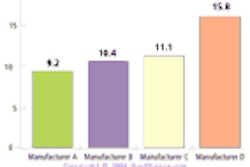
Interventional-device firm Guidant has launched its Voyager rapid-exchange coronary dilatation catheter in the U.S. following receipt of Food and Drug Administration clearance.
Voyager features a number of new technologies, including a low-profile tapered tip, according to the Indianapolis-based firm.
By AuntMinnie.com staff writersJuly 27, 2004
Related Reading
Guidant books record Q2, July 23, 2004
Guidant gets CE Mark, May 27, 2004
Dollens to retire from Guidant helm, May 18, 2004
Guidant notches record Q1, April 22, 2004
Guidant completes BVS acquisition, April 21, 2004
Copyright © 2004 AuntMinnie.com