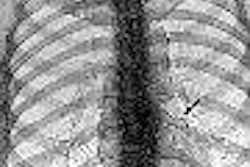
New imaging software company EDDA in Princeton Junction, NJ, has received Food and Drug Administration 510(k) clearance for software for the review of digital x-ray chest images.
EDDA says its software improves the detection rate of lung nodules in the 5-mm to 15-mm range, reducing variation between observers. It delivers multiple image enhancements and region of interest (ROI) evaluation tools, assisting in the identification of pulmonary lesions, according to EDDA.
By AuntMinnie.com staff writers
November 8, 2004
Related Reading
R2 gets FDA nod for lung-nodule CAD, July 12, 2004
X-ray management plan catches lung cancers early, May 28, 2004
Chest x-ray benefits from CAD for spotting lung nodules, March 18, 2004
Lung nodule analysis makes progress, not perfection, March 5, 2004
U.S. panel to consider lung nodule screening tool, February 3, 2004
Copyright © 2004 AuntMinnie.com