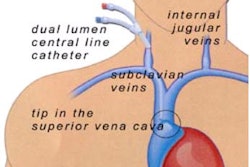
Interventional device developer Boston Scientific of Natick, MA, has submitted the first module of a premarket approval (PMA) application for its Taxus paclitaxel-eluting coronary stent system to the Food and Drug Administration. It is the first of five modules the firm plans to submit in support of the product. The fifth module, expected to be filed in June, will include data from the company’s Taxus IV clinical trials, according to the vendor.
By AuntMinnie.com staff writersFebruary 25, 2003
Related Reading
Boston Scientific posts preliminary 2002 numbers, gets CE Mark, January 22, 2003
Cordis files suit against Boston Scientific, January 15, 2003
Boston Scientific, TriVascular form alliance, January 14, 2003
Boston Scientific biliary stent approved, October 30, 2002
Cook wins German patent fight vs. Boston Scientific, June 6, 2002
Copyright © 2003 AuntMinnie.com