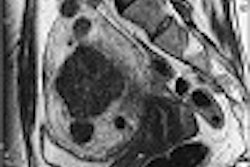
MRI contrast developer Epix Medical of Cambridge, MA, is scheduled to present the phase III clinical results of its MS-325 MRI contrast agent being co-developed by the firm and Schering of Berlin at the annual Scientific Symposium of Transcatheter Cardiovascular Therapeutics (TCT) in Washington DC.
The contrast agent is a currently pending U.S. Food and Drug Administration new drug application (NDA) submission for a vascular disease diagnostic indication. In addition, the firms’ pre-clinical MRI contrast agent designed to image blood clots, EP-2104R, will be the subject of a TCT poster presentation, Epix said.
By AuntMinnie.com staff writersSeptember 15, 2003
Related Reading
Epix exercises overallotment option, August 27, 2003
Epix sets share price, August 7, 2003
Epix to offer more shares, July 28, 2003
Epix shows slight revenue increase, lower loss in Q2, July 25, 2003
Epix completes final phase III trials on MRA contrast agent, July 11, 2003
Copyright © 2003 AuntMinnie.com