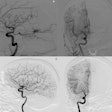
Interventional device developers Boston Scientific of Natick, MA, and EndoTex Interventional Systems of Cupertino, CA, have completed enrollment in a carotid artery stenting clinical trial. The study will evaluate the benefits of stenting in conjunction with embolic protection to treat carotid artery disease.
The Cabernet clinical trial will use the EndoTex NexStent Carotid Stent, and the Boston Scientific FilterWire EZ Embolic Protection System, to treat patients who are at high risk for a carotid endarterectomy (CEA). The study is a single-arm, prospective, non-randomized trial with 450 enrolled patients at high risk for CEA at 15 sites in the U.S.
Both NexStent and FilterWire EZ are limited by U.S. law to investigational use, the firms said. FilterWire EZ has been granted CE Mark and is commercially available in Europe and other international markets, Boston Scientific said.
By AuntMinnie.com staff writersMarch 25, 2004
Related Reading
Boston Scientific gets FDA approval for Taxus, March 5, 2004
Boston Scientific to build intercontinental distribution center, March 4, 2004
Boston Scientific, Guidant settle litigation, February 23, 2004
Boston Scientific gets Canadian approval, February 19, 2004
Boston Scientific expands Minnesota operations, February 18, 2004
Copyright © 2004 AuntMinnie.com