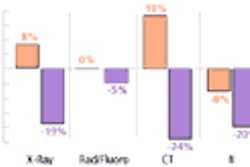
Vascular device developer Conor Medsystems of Menlo Park, CA, has completed enrollment for a clinical trial evaluating its MedStent paclitaxel drug delivery stent.
The trial, Paclitaxel In-Stent Controlled Elution Study (PISCES), is a multi-center, comprehensive dose optimization study involving 190 patients in nine sites across seven countries, according to the company.
The PISCES trial compares the safety and performance of six different formulations of paclitaxel for reducing restenosis in the treatment of native coronary artery lesions. The goal of the study is to determine the optimal release characteristics for paclitaxel in order to minimize angiographic late loss without exhibiting long-term persistence or toxicity of the drug and polymer, according to Conor.
By AuntMinnie.com staff writersMarch 25, 2004
Copyright © 2004 AuntMinnie.com