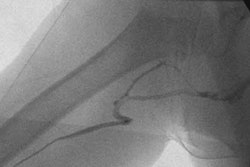
Interventional device developer Abbott Vascular Devices (AVD), a subsidiary of Abbott Laboratories of Abbott Park, IL, has enrolled the first patient in its ZoMaxx I drug-eluting coronary stent clinical trial.
ZoMaxx I is a 400-patient prospective randomized clinical trial that will be conducted in more than 30 centers in Europe, Australia, and New Zealand. The study compares AVD's ZoMaxx drug-eluting coronary stent to the Taxus Express2 drug-eluting stent from interventional device firm Boston Scientific of Natick, MA. The study has a primary end point of nine-month in-segment late loss, a measurement of the narrowing of a vessel.
Enrollment is expected to continue through the first quarter of 2005, the firm said.
By AuntMinnie.com staff writers
September 17, 2004
Related Reading
Abbott debuts StarClose, May 26, 2004
Abbott launches Perclose ProGlide, May 13, 2004
Abbott Labs launches endovascular unit, January 28, 2004
Abbott finishes Jomed deal, July 1, 2003
Abbott facility to produce Adenoscan, March 3, 2000
Copyright © 2004 AuntMinnie.com