Serial F-18 FDG-PET/CT scans show that treatment with infliximab is an effective strategy in patients with refractory cardiac sarcoidosis, according to a study published June 9 in the Journal of Nuclear Cardiology.
The finding is from an observational cohort study and highlights a need for a prospective study to confirm the efficacy of infliximab in managing these patients, noted first author Louise Crowley, PhD, of the University of Birmingham in the U.K., and colleagues.
“This real-world observational cohort study adds to the very limited evidence supporting the use of infliximab in patients with steroid-refractory disease,” the group wrote.
Cardiac sarcoidosis is associated with high morbidity and mortality, with refractive disease defined as disease that persists or progresses despite first-line treatment with corticosteroids, for instance. While a small number of retrospective observational studies have reported that infliximab may be effective in these cases, to date, there are no published randomized controlled trials evaluating the approach.
To build additional evidence, the researchers aimed to examine the efficacy of infliximab for the treatment of steroid-refractory cardiac sarcoidosis using serial quantitative F-18 FDG-PET/CT imaging. From health records, they identified 12 out of 104 patients diagnosed with cardiac sarcoidosis between November 2019 and March 2024 (mean age, 55.2; 58% male) who received infliximab therapy.
Patients received three initial induction doses of infliximab (5 mg/kg) at weeks zero, two, and six, followed by maintenance doses every eight weeks for up to two years. The team quantified treatment response through serial F-18 FDG-PET/CT, with treatment discontinued if there was no active inflammation evident on the scans.
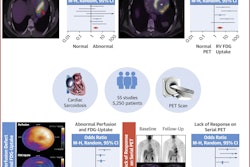
 A graphical abstract of the study.Journal of Nuclear Cardiology
A graphical abstract of the study.Journal of Nuclear Cardiology
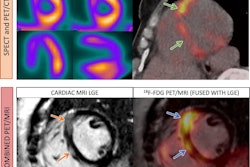
Compared to baseline scans, F-18 FDG PET/CT at a median duration of five months in patients showed reductions in left ventricle (LV) maximum standard uptake values (SUVmax: 13.3 vs. 5.1), LV cardiac metabolic activity (CMA: 400 vs. 0), and right ventricle (RV) SUVmax (3.3 vs. 1.9).
Ten patients had a reduction in CMA, with seven of these having a good response to infliximab based on a CMA reduction rate >70%. In addition, there were discordant findings between visual and semiquantitative assessments: five of 11 patients (46%) at initial follow-up F-18 FDG-PET/CT had focal active cardiac sarcoidosis (SUVmax ≥ 2.5) on semiquantitative analysis despite being initially categorized as negative on visual assessment.
In six patients who have completed two years of infliximab therapy to date, overall findings support a sustained response as determined by F-18 FDG-PET/CT imaging at baseline versus two years (baseline SUVmax 12.3 vs. 3.9). Of these, four were discontinued as per protocol after F-18 FDG-PET/CT imaging, the researchers reported.
“This study highlights a need for a prospective study to confirm the efficacy of infliximab therapy in the management of cardiac sarcoidosis,” the group wrote.
When designing such a trial, it will be important to include comparisons of visual and quantitative assessments of F-18 FDG-PET/CT imaging that incorporate both SUVmax and CMA measurements, they added.
“Nonetheless, these data support that treatment with infliximab in patients with refractory cardiac sarcoidosis represents an effective strategy. Prospective trials are needed to fully inform the benefit of using infliximab in this condition,” the researchers concluded.
The full study is available here.