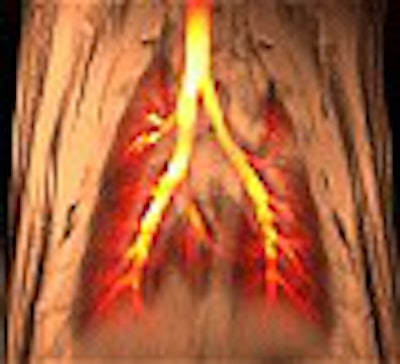
Duke University researchers are reporting the first use of dynamic MRI to simultaneously assess regional lung function and morphology, which they have used to observe what occurs when asthma is induced in small animals.
Although this innovation from Duke’s Center for In Vivo Microscopy (CIVM) in Durham, NC, may be adapted for human clinical imaging, the primary goal is to illuminate the mechanisms of asthma and facilitate improved therapies for a growing epidemic.
"We’re taking the imaging tools that are routine in the clinical arena and making them available to the basic scientists, expecting the same impact on the basic scientists that we’ve seen with clinical imaging in human healthcare," said CIVM director G. Allan Johnson, Ph.D., in an interview with AuntMinnie.com.
CIVM researchers have already seen for themselves what happens when rats are subjected to a methacholine challenge, the standard test for diagnosing asthma in humans. The overreaction of asthmatic lungs to methacholine is usually gauged by measuring the patient’s exhalation; the Duke team could actually visualize the constriction of peripheral airways in rodent lungs, according to their presentation to the 2003 American Thoracic Society meeting in Seattle.
Johnson and his co-authors, Ben Chen, Ph.D., and graduate student Anja Brau, plan to describe their methacholine-challenge findings in detail in a future article for the journal Nature. Meanwhile, a description of their underlying imaging technique has been published recently in Magnetic Resonance in Medicine (January 2003, Vol. 49:1, pp. 78-88).
In order to scan the airways at work, inhaled air is spiked with hyperpolarized helium gas. The Duke researchers then use MR microscopy, with resolution 500 to 10,000 times greater than conventional MRI, to see changes in the hair-like airways of small animal lungs. But microscopy isn’t required for equivalent imaging in humans.
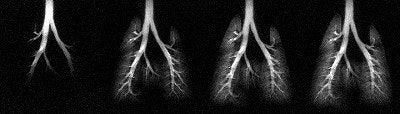 |
| A series of HP 3He images acquired during an inhalation. Using a skip factor = 2 and a flip angle = 12° , more distinct structures of the smaller airways are visible. Each image is displayed in a ventro-dorsal view and was acquired using a 5-mm slice thickness, a 50-mm field-of-view, and 256 x 256 pixels2. |
"It’s easy to scale from mouse to man," said Johnson, noting that clinical researchers are already using hyperpolarized gas imaging based on CIVM’s earlier publications. "I suspect that based on the paper that we hope to see come out in Nature and the paper that has come out in MRM, that there will be variations of those techniques made available for humans."
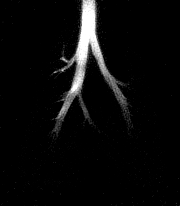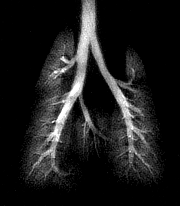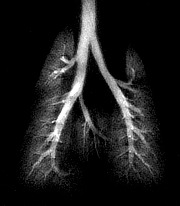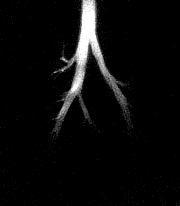 |
| An animated version of the above image. |
According to the MRM, the use of a large flip angle facilitates measurement of regional function in the major airways, while a small flip angle enables measurement in the peripheral airspace.
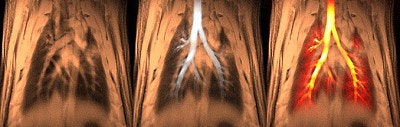 |
| A composite of 1H/3He lung images. Using three different scan schemes demonstrates the structure of the major airways (including the smaller airways), the peripheral airspace, and the thoracic cavity. Images and captions courtesy of CIVM and "Measurement of Regional Lung Function in Rats Using Hyperpolarized 3Helium Dynamic MRI," Chen BT, Brau ACS, Johnson GA, , January 2003, Vol. 49:1, pp.78-88. |
"In the region of the major airways, dynamic changes in SI (signal intensity) during inhalation were shown to match the pattern of the inspiratory airflow (but with a delay), and airflow rate in the ROI (region of interest) was demonstrated to be proportional to the normalized SI in the respective ROI. In the region of the peripheral airspace, the efficacy of regional ventilation was shown to be proportional to the SI in the ROI," the authors wrote.
The researchers also identified air trapping in the lungs by overlaying standard proton MRI and helium MRI scans. Their next effort will use the same imaging methods to study mice that have been genetically altered to have asthma.
CIVM is one of at least 25 federally funded "mouse houses" in the U.S., where research efforts are sponsored by the National Center for Research Resources to generate public information on mouse models of human disease.
"By focusing on these basic models of disease, we are able to get closer to the basic mechanisms that trigger these diseases," Johnson said. "If you want to try to understand asthma in a clinical population, you have a nightmare of complexity just walking in the door. All of the genetic background on which asthma is imposed confuses the hell out of anything you might try to understand."
"On the other hand, if you start with a model where you characterize it well and you know what’s the consequence of environment versus genetic configuration genotype A, environment/genotype B, environment/genotype C, you’re not going to get the genetic information you want in the human population coming in the door," Johnson said. "We’re going to get the answers much more quickly than we could by wading through the clinical epidemiology."
Indeed, CIVM researchers already have hints of where their work will lead.
"We expect that asthma is a very complicated disease with many different mechanisms, many different triggers, many different variations," Johnson said. "By using the approaches we have at our disposal now, we ought to be able to sort out...ones that might be amenable to therapy and ones that might not -- and if they are amenable to therapy, what are the most judicious approaches to those therapies."
By Tracie L. ThompsonAuntMinnie.com contributing writer
July 25, 2003
Related Reading
Polarized helium imaging: an emerging technique to view asthma, July 4, 2003
New MRI method allows visualization of distal airways, April 29, 2003
MRI sizes up airway constrictions in pediatric sleep apnea, January 24, 2003
Thoracic Imaging: Bronchogenic Cyst, April 3, 2002
Copyright © 2003 AuntMinnie.com