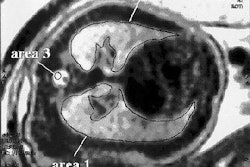
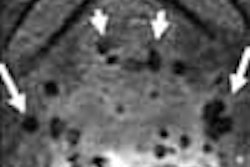
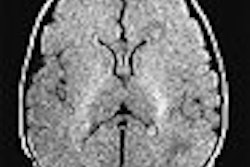
German pharmaceutical firm Schering has submitted Epix Medical 's MS-325 intravenous blood pool MR angiography contrast agent for marketing approval in the European Union.
MS-325, co-developed by Cambridge, MA-based Epix and Schering of Berlin, is designed specifically for vascular imaging in patients receiving MRA, according to the firms. The agent was submitted for U.S. marketing clearance in December 2003.
By AuntMinnie.com staff writers
June 7, 2004
Related Reading
Epix raises $96 million, June 4, 2004
Epix floats new debt, June 2, 2004
Schering gets Swedish approval for Primovist, April 5, 2004
Schering gets European OK for Zevalin for NHL, January 22, 2004
Epix, Schering to partner on MRI contrast, May 27, 2003
Copyright © 2004 AuntMinnie.com