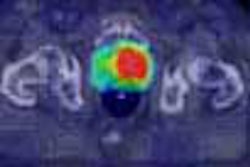
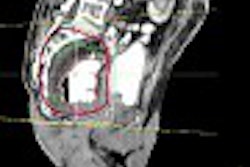
Radiation oncology firm Varian Medical Systems of Palo Alto, CA, has received 510(k) marketing clearance from the Food and Drug Administration for a new single-channel device for delivering high-dose-rate (HDR) brachytherapy.
The MammoSource afterloader is a computer-controlled device that delivers a high-energy radioactive source through a single catheter into the cavity left when a tumor has been surgically removed. The product works with the firm's BrachyVision software, an image-guided HDR brachytherapy treatment-planning system, Varian said.
By AuntMinnie.com staff writersApril 27, 2004
Related Reading
Varian finishes OpTx acquisition, March 30, 2004
Varian to acquire OpTx, March 4, 2004
Varian gets OK for imager accessory, March 3, 2004
Varian posts strong Q1, February 29, 2004
Varian debuts brachytherapy applicator, January 27, 2004
Copyright © 2004 AuntMinnie.com