Hitachi of Twinsburg, OH, will highlight the HI Vision 8500 and HI Vision 6500 ultrasound systems.
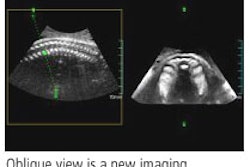
Hitachi is marketing HI Vision 8500 for clinical applications such as elastography, musculoskeletal, abdominal, interventional, ob/gyn, small parts, and vascular imaging, and biopsies.
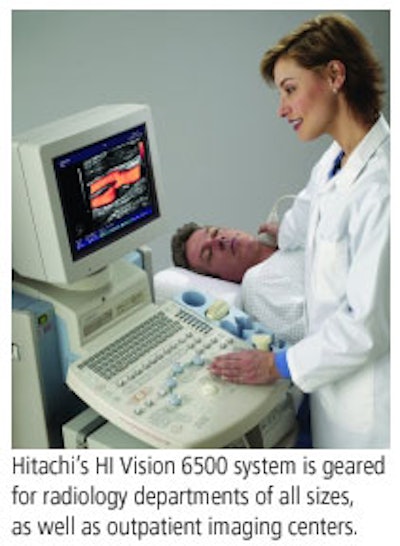
On the HI Vision 6500 model, Hitachi will showcase its new Version 6 upgrade, with new features such as SonoIQ one-touch image optimization and increased imaging depth. Version 6 also supports a group of new options, including automatic intima media thickness measurement and Hitachi's new real-time 3D imaging technique. Primary clinical applications for the 6500 include musculoskeletal, abdominal, interventional, ob/gyn, small parts, and vascular imaging, as well as biopsies.
Both the HI Vision 8500 and 6500 systems are FDA-cleared, and Hitachi is marketing them to outpatient imaging centers and radiology departments of all sizes. The Version 6 upgrade on the 6500 is pending FDA clearance.
By Wayne Forrest
AuntMinnie.com contributing writer
November 9, 2005
Copyright © 2005 AuntMinnie.com