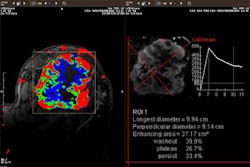
Breast MRI developer Aurora Imaging Technology of North Andover, MA, has received Food and Drug Administration 510(k) marketing clearance for a new, 1.5-tesla version of its dedicated breast MRI scanner. The increase in field strength, from a 0.5-tesla to a 1.5-tesla system, will improve image resolution and is a precursor to the integration of Aurora’s proprietary fat-suppressed, high-resolution, high-contrast imaging technique (RODEO) developed by Dr. Steven Harms and Duane Flamig, according to the company.
By AuntMinnie.com staff writersAugust 7, 2003
Related Reading
Aurora gets OK for interventional breast MRI, February 12, 2003
Aurora scores breast MRI install, November 28, 2002
Aurora reports MRI-guided breast biopsy, September 30, 2002
Copyright © 2003 AuntMinnie.com