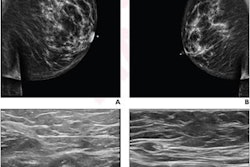
Researchers continue to gain ground in understanding lymphadenopathy caused by the COVID-19 vaccines, and several presenters discussed their findings on the topic on July 13 at ECR 2022.
Three presentations given at the event looked at lymphadenopathy persistence and characteristics, as well as differences in reaction between mRNA and viral vector vaccines.
"Initial clinical management was based on previous experience with other vaccinations, but no strong data was available at the time," said Dr. Yael Adler-Levy from the Hadassah Hebrew University Medical Center in Jerusalem. "With worldwide vaccination implementation, more data has accumulated."
The first COVID-19 vaccines began to be rolled out in late 2020. Since then, various side effects from vaccination have been reported, one of them being the swelling of the lymph nodes, also known as lymphadenopathy.
These swollen nodes can cause concern for radiologists and patients who suspect they could be malignancies on mammography. Societal guidelines in breast imaging advise radiologists to make certain of patient vaccination status to avoid unnecessary follow-up work.
Previous guidelines suggested delaying breast cancer screening if lymphadenopathy occurred, but research suggests that swollen glands can persist up to 12 weeks.
In her presentation, Adler-Levy showed the results of her team's study, which looked at 41 people. The team's goal was to define a timeline to expect lymphadenopathy resolution following vaccination, as well as aid guidelines on deciding to go through with full diagnostic workup.
The researchers found that at six weeks, 52% of the women included in the study achieved resolution. About 30% of the women saw lymphadenopathy persist for more than nine weeks. Biopsies that were performed in cases persisting beyond 12 weeks were found to be all benign, with resolution by the 21-week mark.
"Our data supports current guidelines in preferring follow-up over biopsy as a management option and propose extending the follow-up period," Adler-Levy said. "We recommend deferring biopsy beyond 12 weeks of initial detection."
Another presentation led by Dr. Ignacio Soriano Aguadero from the Navarra University Clinic in Spain showed data that sought to compare the axillary lymph node reaction to COVID vaccination between mRNA and viral vector vaccines.
 In his talk, Dr. Ignacio Soriano Aguadero from the Navarra University Clinic in Spain showed data comparing axillary lymph node reaction to COVID vaccination between mRNA and viral vector vaccines. He and his colleagues found that patients who received the mRNA vaccine had higher values in all ultrasound findings, including Bedi classification grades (pictured).
In his talk, Dr. Ignacio Soriano Aguadero from the Navarra University Clinic in Spain showed data comparing axillary lymph node reaction to COVID vaccination between mRNA and viral vector vaccines. He and his colleagues found that patients who received the mRNA vaccine had higher values in all ultrasound findings, including Bedi classification grades (pictured).Aguadero's team looked at data from 223 people who were given three rounds of ultrasound. This included baseline (before vaccination), first follow-up one week after first vaccination dose, and second follow-up after second vaccination dose.
Out of the total, 146 (66%) of participants received an mRNA vaccine, while the remaining 77 had a viral vector vaccine (AstraZeneca). The team reported significant differences in lymph node characteristics between the two groups.
| Swollen lymph node characteristics in patients who received mRNA and viral vector COVID-19 vaccines | |||
| mRNA | Viral vector | p-values | |
| Average number of lymph nodes | 6.38 | 4.26 | < 0.001 |
| Diameter (mm) | 23.46 | 20.58 | 0.001 |
| Cortical thickness (mm) | 4.81 | 2.92 | < 0.001 |
The group also found a higher frequency of grades four through six on the Bedi classification scale in the mRNA group (61/146) compared with the viral vector group (9/77).
"It seems that these [differences] are related to the way the vaccines act in the axillary lymph nodes and how they're presented to the antibodies," Aguadero said. "We are continuing work."
In another presentation given at the session, Dr. Carola Catanese from the Imaging Institute of Italian Switzerland talked about her team's study that looked at prevalence and time regression of vaccine-related axillary lymphadenopathy in women after breast examination at time intervals. The team also looked at characteristics of swollen nodes in women undergoing breast imaging.
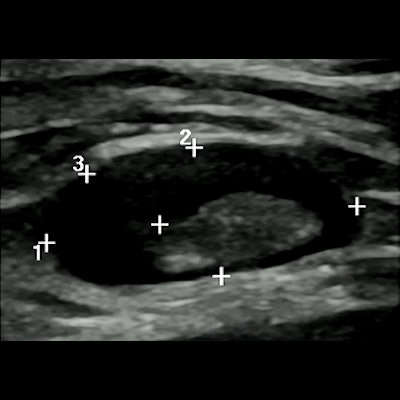
 Dr. Carola Catanese from the Imaging Institute of Italian Switzerland talked at the ECR annual meeting about her team's study on the prevalence, time regression, and characteristics of swollen lymph nodes related to COVID-19 vaccination. Two such characteristics in these patients (pictured) are diffuse or eccentric cortical thickening and partial hilum effacement.
Dr. Carola Catanese from the Imaging Institute of Italian Switzerland talked at the ECR annual meeting about her team's study on the prevalence, time regression, and characteristics of swollen lymph nodes related to COVID-19 vaccination. Two such characteristics in these patients (pictured) are diffuse or eccentric cortical thickening and partial hilum effacement.The researchers analyzed data from 285 women and found that lymphadenopathy or borderline lymphadenopathy occurred in 54 participants (19%). They also found that these cases were more frequent in women who received the Moderna vaccine (p = 0.011). Of the 41 patients with confirmed lymphadenopathy, 20 achieved resolution at six weeks or less. Another 11 had their cases resolved at seven to eight weeks.
Catanese and colleagues also found that women with reported lymphadenopathy were significantly younger than women with borderline or no lymphadenopathy. They added that no significant data was found in women with lymphadenopathy and relation to other clinical data such as previous cancer history (not breast), previous COVID infection, body mass index, and immunosuppressive therapy.
The most common feature found on imaging for women with lymphadenopathy was diffuse or eccentric cortical thickening (53/54 patients), followed by partial hilum effacement (18/54 patients).
Catanese said the team's results support current guidelines, adding that no further assessment should be required.