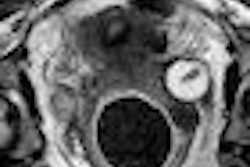
Women's imaging vendor Hologic of Bedford, MA, has received U.S. Food and Drug Administration (FDA) clearance for software designed to assess breast density.
R2 Quantra is a volumetric breast density assessment tool that provides radiologists with an automated method for assessing breast density; it is designed for use with Hologic full-field digital mammography systems. The application creates an internal 3D model of the breast from which it derives estimates of fibroglandular tissue volume and total breast volume. Volumetric breast density is the ratio of these values.
The company believes that Quantra's ability to provide numeric density values for each breast may aid radiologists in assessing breast tissue composition without the subjectivity of human interpretation. Published research indicates that breast density can be almost as powerful a predictor of breast cancer risk as age, according to the company.
Related Reading
Study: APBI compares favorably to standard RT, October 1, 2008
Hologic gets FDA nod for FRAX, September 9, 2008
Cytyc adds to Hologic Q3 revenues, July 31, 2008
Hologic completes tender offer for Third Wave, July 24, 2008
Hologic closer to Third Wave acquisition, July 17, 2008
Copyright © 2008 AuntMinnie.com