French firm eCential Robotics has received clearance from the U.S. Food and Drug Administration (FDA) to market its unified 3D imaging, navigation, and robotics guidance system for use in spine surgery.
The surgical platform unifies intraoperative 2D/3D imaging, navigation, and robotics, according to the company. The system streamlines surgical workflows by automating numerous technical steps, while a single user interface for all devices enables surgeons to control the imaging, navigation, and robotic functionalities from a single input and output graphical display screen.
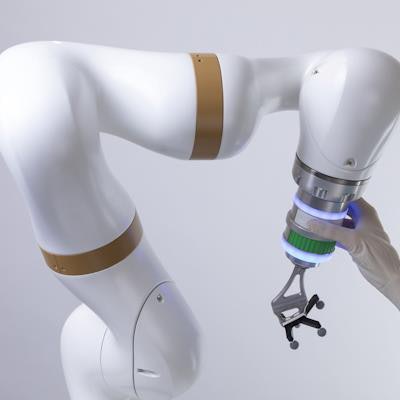
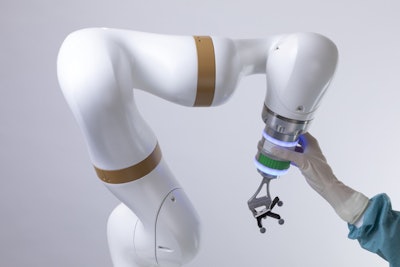 The robotic arm of the surgical robotics platform.
The robotic arm of the surgical robotics platform.The platform has received the CE Mark in Europe, with the first successful commercial launch in France. Since then, 10 units have been sold and installed in Europe and more than 2,000 surgeries have been performed using the system, eCential said.
A team from eCential Robotics will be demonstrating the system at the upcoming Society for Minimally Invasive Spine Surgery 2022 annual meeting and the North American Spine Society 2022 annual meeting in Chicago in coming weeks.