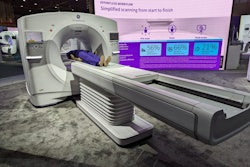
GE HealthCare (GEHC) has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for a new version of Digital Expert Access with remote scanning.
Digital Expert Access allows for the sharing of expertise, best practices, and in-the-moment advice, as well as real-time remote console control.
In related GEHC news, the company has entered into an exclusive distribution agreement with Ionic Health, an independent Brazilian-based global technology company specializing in automation technologies. This includes remote healthcare tools to automate, monitor, access, support, educate and teleoperate in healthcare. Ionic's 510(k)-pending Command Lite technology for remote scanning.
GE HealthCare said the Ionic agreement will allow it to provide multi-vendor, multi-modality remote scanning technology to healthcare systems and patients worldwide.