Updated October 4, 2002
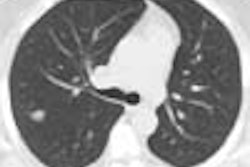
The Food and Drug Administration has become aware of a possible association between cochlear implants and the occurrence of bacterial meningitis. Worldwide, the agency knows of 91 reports of meningitis in patients implanted with the three FDA-approved cochlear implant devices: Advanced Bionics Corporation devices (56 cases), Cochlear Limited devices (33 cases) and MED-EL Corporation devices (2 case). Seventeen deaths have resulted from these meningitis cases.
FDA public health notification: Cochlear implant recipients at greater risk for meningitis
Oct 3, 2002
Latest in Industry News
Median names Oran Muduroglu to lead U.S. eyonis LCS launch
February 19, 2026
KU Health, BAMF Health to build adult-pediatric theranostics hub
February 19, 2026
Positrigo program to bring brain PET to U.S. neurology offices
February 19, 2026