CHICAGO - A new 256-slice CT scanner, a full-field digital mammography system, and a vision of what the reading room of the future might look like are among the highlights in the RSNA booth of Philips Medical Systems of Andover, MA.
CT
Brilliance iCT represents Philips' entry in the ongoing CT slice wars. The 256-slice system is based on the company's new Essence technology platform, which includes technology advances in x-ray tube design, detector materials, and reconstruction elements.
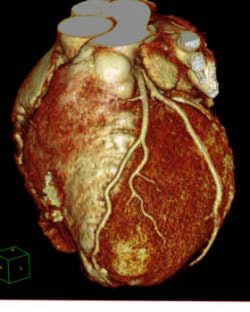 |
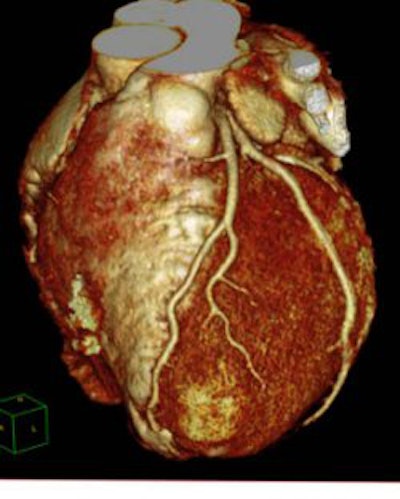
| A cardiac image from Philips' Brilliance iCT 256-slice scanner. |
Philips earlier in November shipped a beta version of the Brilliance iCT scanner to MetroHealth System in Cleveland, where it's being used on an investigational basis. Philips believes the scanner will be especially useful for cardiac studies, thanks to its 270-msec gantry rotation speed. The company estimates that commercial deliveries of Brilliance iCT will begin in 2009.
Philips has also migrated Essence technology to its 64-channel scanner, and 30 new Brilliance 64 scanners have already been shipped with the upgrade. Essence 64-slice scanners are now available in two configurations: an economy-level system that has a list price comparable to a 40-channel scanner, and a fully featured system optimized for cardiac studies.
Philips is also touting new workstation enhancements, such as a new treatment analysis package, and the company's Brilliance Everywhere thin-client portal. The company is also developing what it calls Spectral CT, a technique in which CT is used to image at multiple energy levels to identify specific chemical elements in the human body.
PACS/healthcare informatics
Philips is demonstrating the latest versions of its iSite Radiology and iSite Enterprise PACS with advanced visualization and mammography support tools.
The iSite PACS' advanced visualization tools are designed for on-demand images over existing hospital networks, with advanced radiology reading stations for radiologists and long-term storage.
Philips is also introducing iSite Volume Vision, a 3D visualization toolset for clinical applications, and Xcelera R2.1, a multimodality cardiology information management technology making its RSNA debut.
Xcelera R2.1 is designed to organize and provide access to images and data from cardiology subspecialties, including echocardiography, cardiovascular ultrasound, and electrophysiology.
In addition, Philips is drawing attention to its Reading Room 20/20 environment, a concept development that takes the firm's Ambient Experience to a new level. Reading Room 20/20 offers a vision of a future reading room in which radiologists use large touch-screen medical displays, noise-canceling technologies, and wall-sized videoconferencing displays to read, communicate, and transmit medical images.
MRI
Among the highlights in the MRI section of Philips' booth is MammoTrak, a new breast MRI capability. Shown as a work-in-progress, MammoTrak includes two specialized breast imaging coils, a dedicated breast MRI table, and analysis software. The technology will be available as an upgrade to Philips MR customers, and will begin shipping at the end of 2008.
Other technologies being showcased in the company's booth include an A-series version of the Achieva 1.5T platform. The product now includes Philips' SmartExam protocols and 16-channel gradients as standard features, as well as a greater range of dedicated high-channel coils and parallel imaging acceleration.
The X-series upgrade for the Achieva 3.0T scanner includes magnet, gradient, and radiofrequency technology for better performance and an enhanced field-of-view. A mobile version of Achieva 3.0T X-series offers the same functionality as the stationary version.
Advanced MR applications that the company is touting include 4D-TRAK MR angiography, a time-resolved angiography technique using keyhole imaging; FiberTrak for visualization of white-matter fibers and neuro fiber bundles; k-t BLAST, a cardiac MR technique; 2k imaging over a large field-of-view; and spectroscopy with the company's SENSE (sensitivity encoding) parallel imaging technique.
X-ray
Philips' x-ray division has caught the wireless bug, demonstrating a wireless digital detector plate, Eleva Wireless, integrated into two radiography systems: the company's DigitalDiagnost radiography system and Practix Covenio DR mobile digital radiography (DR) unit.
The plate is based on a digital detector from Trixell of Moirans, France, and includes built-in technology that enables image transmission to a central computer in a few seconds for a standard radiography study. Philips is demonstrating the wireless capability as a work-in-progress, and expects the technology to be commercially available next year.
Another Philips x-ray highlight is the rollout of its Eleva user interface to other systems in the company's x-ray division. Initially used on the company's computed radiography systems, Philips believes that making Eleva available on DR units as well will reduce technologist training requirements and improve productivity.
Philips is also featuring DRF, a room-based DR system that supports both digital radiography and fluoroscopy imaging. It combines the EasyDiagnost Eleva system with a choice of either a digital vertical stand and/or a table-based digital detector.
Finally, Philips is touting its xLNA Enterprise computer-aided detection (CAD) capabilities available for lung DR studies, made available through its partnership with EDDA Technology of Princeton Junction, NJ.
In cardiovascular x-ray, Philips is promoting the next generation of its Allura Xper interventional imaging systems. The new Allura family is designed to support all interventional imaging procedures, from neuro to abdominal to peripheral. New enhancements include a new patient table, integrated 3D interventional tools, and advanced positioning capabilities available at the system's tableside controls.
Philips is also highlighting new versions of its XperCT soft-tissue imaging product, and 3D guidance tools, including Dynamic 3D Roadmap and XperGuide, for helping guide needles and catheters through complex anatomy.
Mammography
 |
| MammoDiagnost DR is a work-in-progress. |
MammoDiagnost DR features a large (24 x 30-cm) field-of-view, and includes Philips' Unique image processing software and Eleva workflow package. The company expects MammoDiagnost DR to be available in 2008.
Ultrasound
Philips is showing upgrades to its iU22 Vision 2008 ultrasound system, including the new C5-1 PureWave transducer for the iU22. The curved-array device is designed for abdominal, ob/gyn, and interventional applications for patients who are difficult to image. Philips' PureWave technology adds new advances in tissue aberration correction, coded beamforming, and advanced XRES technologies to the C5-1.
Philips' ViewForum standalone workstation has also received an upgrade with a range of 3D review capabilities for radiology departments and imaging centers with multimodality PACS networks.
In addition, Philips has added the BP10-5ec broadband biplane transducer for prostate imaging to its HD11 XE ultrasound system.
Molecular imaging
Among the highlights in nuclear medicine is Philips' work-in-progress Big Bore PET/CT system, which combines the company's Brilliance CT Big Bore and Gemini TF PET scanners. Designed for radiation oncology and interventional radiology applications, the system features a bore diameter of 85 cm for both PET and CT.
 |
| Philips is highlighting its Big Bore PET/CT system in its RSNA booth. |
The company anticipates that the new system will be available in late 2008 or early 2009.
Philips also showed work-in-progress features for its BrightView SPECT variable-angle camera, with an aperture of 38 inches for larger patients. Under development are features that include co-planar SPECT and CT; Volume CT, which uses flat-panel, low-dose x-ray; a variable axial slice of 0.3 mm to 2 mm; and image acquisition by either 12-second breath-hold or tidal respiration of 60 seconds. Philips anticipates availability in late 2008 or early 2009.
Another work-in-progress is Philips' PET/CT Viewer version 2.0 for the Brilliance Workspace to review and analyze images for routine clinical evaluation of PET/CT examinations.
Version 2.0 expands the viewing layout and offers multimodality fusion for automated registration using data-specific algorithms. Tumor tracking and follow-up provides physicians with updates on the efficacy of therapy.
Also under development is AutoQuant 7.0, a cardiac quantification package from Cedars-Sinai Medical Center in Los Angeles. AutoQuant 7.0 incorporates Astonish and Vantage normals, prone supine imaging, stress test registration, and SPECT and PET quantification and CT angiography with perfusion overlay.
By Brian Casey and Wayne Forrest
AuntMinnie.com staff writers
November 27, 2007
Related Reading
Philips, Elsevier to partner on speech recognition, November 15, 2007
Road to RSNA, Healthcare Informatics, Philips Medical Systems, November 8, 2007
Road to RSNA, Ultrasound, Philips Medical Systems, November 5, 2007
Philips, ImaRx ink research deal, November 5, 2007
Road to RSNA, Medical Displays, Philips Medical Systems, November 2, 2007
Copyright © 2007 AuntMinnie.com