Brain SPECT Imaging in Dementia:
Alzheimer's Dementia:
Dementia affects 10% of people over the age of 60 years and Alzheimer's accounts for roughly 50% of these cases. Alzheimer's disease (AD) has a prevalence of 0.3% in patients aged 60-69 years, but increases to nearly 11% in 80-89 year olds [2]. The mental degeneration associated with Alzheimer's is insidious and progressive memory loss is the most important symptom [2]. Transient stabilization of cognitive and behavior function can be achieved with acetylcholinesterase inhibitor therapy [6].
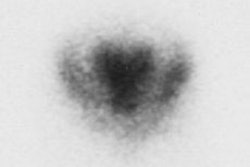
Dementias produce deficits in perfusion, in part reflecting decreased metabolic needs. In Alzheimer's one classically sees bilateral decreased metabolism (PET imaging) and flow (SPECT imaging) in the temporal and parietal lobes with sparing of the primary motor, sensory, and visual cortices. The temporoparietal defects are noted in about 65% of Alzheimer's patients and are the most consistently recognizable sign of Alzheimer's, particularly when relatively symmetrical (although symmetric, the defects are not necessarily of the same magnitude and severity [2]). This pattern has a predictive value of over 80% for the diagnosis of Alzheimer's disease. A correlation has also been described between the severity of these defects, and the severity of the patient's dementia. Unilateral temporoparietal or isolated frontal defects can be found in up to 20% of Alzheimer's patients, and are more commonly seen early in the disease process. When the medial temporal lobes are particularly abnormal, the memory deficit is often more marked and reflects the functional importance of this region for memory. Frontal lobe abnormalities may also be seen in cases of advanced disease, but are less specific for Alzheimer's. The cerebellum, primary visual areas, and primary sensorimotor areas along the central sulcus remain relatively intact. Perfusion defects identified on SPECT images have been shown to correlate histologically to areas that contain large amounts of plaques and neurofibrillary tangles. In the later stages of the disease process, cortical atrophy itself contributes approximately 20% of the decrease in activity seen in the temporoparietal areas, possibly secondary to volume averaging.
Overall, Tc99m-HMPAO imaging has a sensitivity between 80-90% and a specificity between 65-87% for the diagnosis of Alzheimer's dementia. The negative predictive value of a normal study is approximately 80% (ie: about 20% of patients affected with Alzheimer's disease may have a normal CNS perfusion exam) [1,2]. In one study which compared SPECT exam findings with autopsy results, SPECT imaging had a sensitivity of 63%, a specificity of 82%, a positive predictive value of 81%, a negative predictive value of 65%, and an accuracy of 71% for the diagnosis of Alzheimer's (which was comparable to the clinical assessment for the disorder) [4]. This same study found that the greatest utility of SPECT imaging was in increasing the confidence of the clinical diagnosis for AD [4]. When SPECT imaging findings and clinical diagnosis for Alzheimer's disease are considered together, a positive SPECT interpretation increases the odds of having Alzheimer's by an additional factor of five [4]. SPECT imaging also holds potential for monitoring response to medical therapy [6]. Regional cerebral perfusion appears to remain stable in patients with stabilization of cognitive function while receiving acetylcholinesterase inhibitor therapy [6].
Other disorders to be considered in the differential of
biparietal/temporal defects include: Parkinson's disease with
dementia, bilateral parietal infarcts, multi-infarct dementia
(typically asymmetric, and multiple small cortical defects),
vertebral basilar insufficiency (produces bilateral occipital
perfusion abnormalities which should be distinguished from the
parietal abnormalities associated with Alzheimer's), hypoglycemia,
and carbon monoxide poisoning. In a small percentage of patients,
a combination of multi-infarct and Alzheimer's dementia may
coexist (mixed dementia).
Parkinson's Disease:
There are four major dopaminergic pathways in the brain [16]. The
largest of the four is the nigrostriatal pathway which contains
about 80% of the brains dopamine and is composed of neurons that
originate in the substantia nigra of the mid brain and terminate
in the caudate and putamen- collectively termed the striatum [16].
If 60-70% of the nigrostriatal pathway cells are lost because of
disease, the classic symptoms of parkinsonian motor dysfunction
develop (resting tremor, rigidity, postural instability, and
bradykinesia or akinesia [16]. Parkinsonian syndrome is an
umbrella term for a group of neurologic disorders that occur due
to nigrostriatal degeneration and include idiopathic Parkinson
disease (comprises 75% of Parkinsonian syndrome cases),
multisystem atrophy, progressive supranuclear palsy,
corticobasilar degeneration, diffuse Lewy body disease, and
vascular Parkinson disease [16].
Idiopathic parkinson disease is a progressive neurodegenerative
disorder resulting from the progressive death of dopaminergic
neurons in the nigrostriatal pathway [5]. It occurs in 1% of the
population older than 55 years of age [7]. The median age at
diagnosis is 60 years, although 5-10% of patients present before
age 50 years, and the average duration of disease is 15 years from
diagnosis to death [16]. Men are affected 1.5-2 times more
frequently than women [16]. There has been a significant increase
in incidence over the past 30 years, particularly in men over the
age of 70 years [16].
Symptoms consist of rigidity, bradykinesia, difficulty in initiating and stopping movement, and a resting tremor [5]. Motor disturbances begin only after a loss of approximately 70-80% of striatal dopamine- thus, there is a long latent period which precedes the development of clinical symptoms [5]. Approximately 10% of patients affected with Parkinson's disease will develop dementia [2]. Generally, the perfusion pattern in these patients in non-specific and demonstrates either normal or mild global cortical deficits. A pattern of bilateral posterior parietal/temporal defects indistinguishable from Alzheimer's may be observed in patients with Parkinson's disease with dementia [2].
Other agents used for imaging Parkinsons include 123I-FP-CIT
(which
images
dopamine
transporter
binding
and presynapic dopaminergic degeneration of the nigrostriatal
tract), 123I-IBZM (which images dopamine D2
receptors), and 123I-MIBG that can demonstrate
pronounced cardiac sympathetic denervation in patients with
Parkinsons disease (however, there is considerable overlap between
Parkinsons and Atypical Parkinsons Disorder and low MIBG uptake
alone does not necessarily indicate PD) [8].
123I-ioflupane:
The agent 123I-FP-CIT or 123I-ioflupane
(N-w-fluoropropyl-2?-carbomethoxy-3?-(4-123I-iodophenyl)
nortropane)
is
a
molecular
analog of cocaine that binds reversibly with high affinity to the
dopamine transporter protein (DaT) [9,12]. DaT is a transmembrane
protein in the presynaptic membrane of the dopaminergic synapse
that transports dopamine from the synaptic cleft back into the
presynaptic neuron [9]. Dopamine transporters function to clear
and recycle dopamine from the synaptic cleft located in the
putamen and caudate nucleus [13]. The agent can be used to
demonstrate the location and concentration of dopamine
transporters (DaTs) in the presynaptic terminals of striatal
dopaminergic nerons [9,12]. The reduction of tracer binding is
associated with the integrity of the nigrostriatal pathway- namely
dopaminergic neuronal density and axonal dysfunction [17].
123I-ioflupane can aid in separation of patients with
essential tremor from those with presynaptic Parkinsonian
syndromes by identifying a presynaptic dopaminergic deficit [9].
DaT concentrations are lower in the striatum (particularly the
putamen) in presynaptic Parkinsonian syndromes (even in early
stage disease) and will be normal in patients with essential
tremor, drug induced parkinsonism, and psychogenic parkinsonism
[9,15]. DaT concentrations are also normal in patients with
Alzheimers dementia, but are significantly reduced in patients
with dementia with Lewy bodies [9], as well as in patients with
progressive supranuclear palsey [15].
Ioflupane is an analog of cocaine. Medications that interfere
with 123I-ioflupane binding should be discontinued for
at least 5 half-lives [9]. Cocaine, amphetamines, and
methylphenidate severely decrease 123I-ioflupane
binding to DaT [9,16]. The CNS stimulants used for appetite
supression ephedrine and phentermine may also decreased 123I-ioflupane
binding [9,16]. Bupropion (used for smoking cessation), fentanyl,
and some anesthetics (ketamine, phencyclidine, and isoflurane) may
decrease 123I-ioflupane binding, as well [9,16].
Cholinesterase inhibitors and neuroleptics probably do not
interfere significantly with binding [9].
Anti-Parkinson drugs in standard doses such as levodopa,
l-dihydroxyphenylalanine, dopamine agonists, monoamine oxidase B
inhibitors, N-methy-D-aspartate receptor blockers, amantadine, and
catechol-O-methyltransferase
inhibitors in standard doses, do not interfere with binding to any
significant degree and do not need to be stopped [9]. A caveat to
this is that long term usage of levodopa can down-regulate the
expression of DaT on the striatal presynaptic neurons [16].
Selective serotonin reuptake inhibitors used in anxiety and
depression may increase binding to DaT somewhat, but should not
interfere with exam interpretation [9,16]. It should be noted that
certain medications - selegiline, sertraline, citalopram, and
paroxetine can significantly affect the scan [13].
There is a normal decrease in DAT striatal binding of about 5-7%
per decade, but this decrease is small in comparison with
decreases caused by disease and normally should not interfere with
exam interpretation [16].
Examination:
The injection may contain up to 6% of free 123I [16]. Thyroid blocking using a single 400 mg dose of potassium perchlorate or a single dose of potassium iodide oral solution or Lugol's solution (equivalent to 100 mg of iodide) at least one hour prior to tracer injection may be considered, but even without blocking, the dose to the thyroid gland from the agent is low [9].
The dose is typically 5 mCi (185 MBq) given via a slow (20
second) IV infusion [9]. Patients do not need to be kept in a dim
or quiet environment prior to injection [9]. Imaging is usually
started 3-6 hours after tracer injection [9,16].
For imaging, the photopeak should be set for 159 keV +/- 10% and
a high-resolution collimator should be used [9,16]. The field of
view for imaging should include the brain and have the smallest
possible circular rotational radius possible- typically 11-15 cm
[9]. A 128 x 128 matrix is recommended for a pixel size of 3.5-4.5
mm [9]. Images can be acquired using a step-and-shoot mode with
angle increments of 3 degrees or using a continuous rotation [9].
The number of seconds per head position is usually 30-40 seconds
and a minimum of 1.5 million counts should be collected for
optimal images [9,16]. However, this may be challenging in
severely abnormal scans with minimal striatal activity [16].
Because 123I-ioflupane is excreted by the kidney, patients with severe renal impairment may have increased radiation exposure and altered 123I-ioflupane imges [9].
Exam findings:
The exam is particularly susceptible to motion artifacts [16].
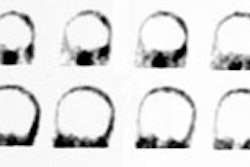
The normal exam should demonstrate homogenenous, well-defined
tracer uptake within the striata that should appear as two
symmetric crescent or comma-shaped regions of focal activity
corresponding to the paired caudate and putamen on transaxial
images [9,16]. Mild asymmetry between left and right may occur in
healthy individuals [16]. A normal exam would be expected in
patients with essential tremor, dystonic tremor, drug-induced
Parkinsonism, medication or drug induced tremor, most vascular PD,
Alzheimers, psychogenic tremor, and in healthy individuals [16].
Abnormal striata will have reduced intensity on one or both sides, often shrinking to a circular or oval shape [9]. Other authors state that in Parkinson disease DAT imaging typically shows asymmetric reduction, predominantly in the striatum contralateral to the clinically most affected side [17]. Caudate and putaminal activity should be compared- generally the putamen, in particular the posterior putamen, is affected earlier and to a greater degree than the caudate in patients with Pakinsonism [13]. This may frequently be asymmetric, particularly in the early stages of disease, with a more pronounced loss in the striatum contralateral to the clinically affected limbs [16]. Abnormal studies typically fall into one of three appearances: asymmetric decreased putaminal activity (this produces a comma-shaped focus on one side and a period-shaped focus on the other side; absent putaminal activity, but preserved caudate activity (two period shaped foci of activity); or absence of putaminal activity and greatly reduced activity in one or both caudate nuclei [13,16].
There are a few pitfalls in exam interpretation- the most common
is the "semicolon sign" secondary to forward head tilt [16]. This
results in the caudate nuclei being seen on separate axial slices
from the putamen and gives the false impression that DaT activity
in the putamenis decreased or absent [16]. By scrolling up and
down through the striata, it will be apparent that there is normal
intense activity in these structures [16]. Another SPECT artifact
is the "pinwheel sign" which arises from rotational head motion
during exam acquisition [16]. The "kissing caudate sign" occurs
when left and right caudate appear fused without an intervening
gap- this occurs secondary to either patient motion or by using
too large a radius for the SPECT exam [16].
Intense activity outside the brain included in the FOV can result
in a scaling artifact [16]. If intense uptake in the salivary
glands is included in the FOV, it can result in striatal activity
being scaled down on reconstruction images [16]. Striatal activity
may appear absent resulting in a false positive exam [16].
123I-ioflupane has been shown to have a sensitivity and specificity (exceeding 90%) and accuracy in differentiating between Parkinson disease and essential tremor [13,17]. Image quantification can improve diagnostic accuracy [15]. DAT imaging can lead to a change in diagnosis in 31-50% if patients and changes in management for 52-58% of patients [16]. However, about 11% of patients with Parkinsonian syndrome can have normal DAT imaging [16]. The patients have been shown to behave differently from subjects with classic PD in that they lack an appropriate response to medication [16]. They also show little or no progressive decline of motor function and their prognosis is more favorable [16].
Drug-induced Parkinson (DIP):
DIP may develop in individuals treated with dopamine
receptor-blocking agents, such as neuroleptics and antiemetics,
dopamine depleters, and calcium channel blockers [14]. DIP
symptoms usually disappear within 2 months of discontinuation of
the fofending drug [14]. DAT imaging is normal in patients with
DIP because the condition occurs in the absence of presynaptic
dopaminergic deficits [14].
123I-MIBG in Parkinsons disease:
Reduction of MIBG uptake in the heart (cardiac sympathetic
denervation) is considered a specific finding for idiopathic
Parkinson's disease without autonomic failure and can be used to
differentiate it from other Parkinsonian
syndromes [10]. The pathobiologic mechanism appears to be
neurodegenerative, associated with alpha-syniclein accumulation in
the autonomic cardiac plexus [11]. However, other authors
indicated that there is considerable overlap between Parkinsons
and Atypical Parkinsons Disorder and low MIBG uptake alone does
not necessarily indicate PD [8]. Although sympathetic function
appears globally reduced on planar MIBG imaging, 11C
Hydroxyephedrine PET imaging suggests the denervation to be
segmental, involving the proximal lateral LV wall most severely,
and with relative sparing of the anterior and proximal septal
walls [11].
Multi-infarct Dementia:
Multi-infarct dementia (MID) is characterized clinically by multiple cerebral infarcts that occur sporadically and produce a step-wise deterioration in intellectual function. MID is the second most common cause of dementia in the elderly [2]. HMPAO findings that suggest the diagnosis include multiple, bilateral, and randomly distributed cortical perfusion defects that follow vascular territories. The basal ganglia, motor, and sensory cortices may also be involved (spared in Alzheimer's).
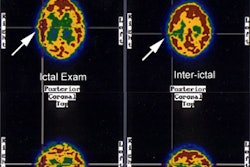
Ischemic brain disease refers to dementia secondary to ischemia without evidence of frank infarction. The Diamox challenge test can be used to identify areas of perfusion abnormality in these patients as their baseline exam is often normal.
HIV:
AIDS dementia complex (ADC) can be seen in up to 11 to 65% of HIV patients. Multiple areas (small and large) of decreased perfusion are identified in the cortical and subcortical regions, often producing a patchy distribution of the tracer. Basal ganglia involvement is also common. The number of defects identified does not necessarily correlate with the severity of the patients' symptoms or clinical findings. The perfusion pattern may improve with therapy (AZT, Calcium channel blockers). SPECT imaging has about a 90% sensitivity in the detection of ADC. The MRI findings in these patients range from normal, to atrophy, to multiple focal white matter signal abnormalities.
Scintigraphic findings similar to ADC have also been described in cocaine polysubstance drug abusers, Lyme disease, and chronic fatigue syndrome. In polysubstance drug abusers the defects are felt to be related to the vasospastic actions of cocaine. Although improvement can be seen following abstention or treatment with buprenorphine, many of the defects are permanent. A recent study [3] suggested that the improvement in perfusion was related primarily to treatment with buprenorphine, was dose dependent, and again worsened upon removal of the drug.
Patients with vasculitis secondary to Lyme disease may
demonstrate SPECT findings indistinguishable from ADC, including
basal ganglia involvement. The scan will improve in these patients
following successful antibiotic therapy.
Pick's Disease (Frontal lobe dementia):
A rare frontal dementia characterized by bilateral cerebral degeneration with atrophy affecting the frontal or temporal lobes and involving both gray and white matter [2]. Symptoms usually include the gradual onset of confusion with respect to place and time, anomia, slowness of comprehension, loss of tact, and changes in personality and behavior [2]. SPECT images demonstrate bilateral, diffuse decreased frontal lobe perfusion extending to the cingulate gyrus [2].
Progressive supranuclear palsy:
PSP is characterized by parkinsonism with bradykinesia and rigidity, postural instability, and a pseudobulbar syndrome with dysarthria and dysphagia [13]. The key feature of PSP is supranuclear palsy of vertical gaze (although this may be absent at disease onsset) [13]. In contrast to Parkinson disease, PSP manifests as a symmetric rather than asymmetric-rigid syndrome [13]. PSP also initially targets the trunk and neck, rather than the limbs, causing early postural and gait instability with falls [13]. The condition will also produce decreased activity within the frontal lobes. FDG PET reveals decreased glucose metabolism in the basal ganglia, midbrain, and mindline frontal lobes- in particular, the anterior cingulate gyrus [13]. There is no cortical deposition of amyloid on amyloid PET imaging [13]. 123I-ioflupane demonstrates reduced striatal dopaminergic activity with variable patterns that may be symmetric or asymmetric and may affect the putamen or caudate nucleus [13].
Huntingtons disease:
Huntingtons disease is an autosomal dominant, degenerative movement disorder characterized by chorea, dementia, and psychiatric symptoms [2]. Brain SPECT imaging will demonstrate decreased or absent tracer uptake in the caudate or basal ganglia [2]. The defects are usually bilateral, but are not necessarily symmetric [2].
Korsakoff's Syndrome:
Seen in alcohol abusers, it is due to thiamine deficiency. Perfusion defects tend to involve the posterior cortical regions predominantly.
REFERENCES:
(1) J Nucl Med 1992; Holman B, et al. Functional brain SPECT: The
emergence of a powerful clinical method. 33: 1888-1904
(2) J Nucl Med 2001; Camargo EE. Brain SPECT in Neurology and Psychiatry. 42: 611-623
(3) J Nucl Med 1995; Levin J, et al. Improved regional cerebral blood flow in chronic cocaine polydrug users treated with buprenorphine. 36: 1211-15
(4) Neurology 2001; Jagust W, et al. SPECT perfusion imaging in the diagnosis of Alzheimer's disease. A clinical-pathologic study. 56: 950-956
(5) J Nucl Med 2001; Huang WS, et al. Evaluation of early-stage Parkinson's disease with 99mTc-TRODAT-1 imaging. 42: 1303-1308
(6) J Nucl Med 2002; Nobili F, et al. Brain perfusion follow-up in Alzheimer's patients during treatment with acetylcholinesterase inhibitors. 43: 983-990
(7) J Nucl Med 2003; Prunier C, et al. Quantificaiton of dopamine
transporter by 123I-PE2I SPECT and noninvasive logan graphical
method in Parkinson's disease. 44: 663-670
(8) J Nucl Med 2011; S?dmeyer M, et al. Diagnostic accuracy of
comboned FP-CIT, IBZM, and MIBG scintigraphy in the differential
diagnosis of degenerative Parkinsonism: a multidimensional
statistical approach. 52: 733-740
(9) J Nucl Med 2012; Djang DSW, et al. SNM practice guideline for
dopamine transporter imaging with 123I-ioflupane SPECT
1.0. 53: 154-163
(10) J Nucl Cardiol
2008; Henneman MM, et al. Cardiac
neuronal imaging: application in the evaluation of cardiac
disease. 15: 442-55
(11) Radiology 2012; Wong KK, et al. Pattern of Cardiac
Sympathetic Denervation in Idiopathic Parkinson Disease Studied
with 11C Hydroxyephedrine PET. 265: 240-247
(12) J Nucl med 2014; Grosset DG, et al. Safety nalysis of 10
clinical trials and for 13 years after first approval of Ioflupane
123I injection (DaTscan). 55: 1281-1287
(13) Radiographics 2014; Broski SM, et al. Structural and
functional imaging in Parkinsonian syndromes. 34: 1273-1292
(14) Radiology 2016; Sung YH, et al.
Drug-induced Parkinsonism versus idiopathic Parkinson disease:
utility of Nigrosome 1 with 3-T imaging. 279: 849-858
(15) J Nucl Med 2017; Booij J, et al.
Diagnostic performance of the visual reading of 123I-ioflupane
SPECT images with or without quantification in patients with
movement disorders or dementia. 58: 1821-1826
(16) AJR 2019; Banks KP, et al. Optimizing
the diagnosis of Parkinsonian syndromes with 123I-ioflupane
brain SPECT. 213: 243-253
(17) Radiology 2021; Villemagne VL, et al. Molecular imaging approaches to dementia. 298: 517-530