SPECT Brain Perfusion Scintigraphy:
Clinical Value/Basic Principles:
Brain SPECT imaging provides complementary functional information to anatomic imaging exams [8]. SPECT agents such as Tc-HMPAO and Tc-ECD reflect cerebral perfusion. Oxygen and glucose are provided to each cerebral region according to its metabolic need [8]. Activated neurons have increased glucose consumption, but have only a limited ability to store glucose [12]. Therefore, increased cerebral blood flow is needed to deliver the glucose required for increased metabolic needs- hence, cerebral blood flow (CBF) is coupled to neuronal activity [12]. Cerebral perfusion and metabolism are coupled in most physiologic conditions, with a few exceptions such as subacute stroke and some brain tumors [8]. The parallelism between CBF, metabolism, and neuronal activity is the basis for the use of brain perfusion SPECT imaging in detecting cerebral dysfunction [8].
The normal adult brain shows bilateral symmetric tracer distribution [9]. Normally, the visual cortex of the occipital lobes and the cerebellum are clearly evident as the areas of most intense activity. Midline structures including the basal ganglia and thalami should be slightly less intense, but clearly evident and relatively symmetric. Eyes open or closed may increase or decrease, respectively, the visual cortex activity by 30% [9]. With aging, there is an overall decrease in brain metabolism which is usually most pronounced in the frontal lobes, while the temporal and parietal lobes are less affected. There is no significant change identified within the subcortical nuclei, calcarine cortex, or the cerebellum with aging. In newborns, blood flow to the frontal and temporoparietal regions is slightly decreased and the greatest cerebral metabolism is in the sensorimotor cortex, thalamus, mid brain, brain stem, and cerebellum. By 3 months of age, activity should be seen in the basal ganglia and associated cerebral cortex. By 2 years of age, the cerebral pattern of metabolism should be similar to adults with normal frontal lobe activity [9].
A controversial issue is what magnitude of left-right asymmetry should be considered clinically significant. Some advocate a threshold value of greater than 10% (visual assessment can detect cortical defects equal to approximately 10% or more of the normal cortical activity), however, biologically significant differences may exist at a lower level. A more general criteria may be a rCBF asymmetry that shows a significant difference from other cortical areas [1]. Other authors consider a cortical perfusion defect an area at least 1 cm in diameter, with less than 60% of the level of activity in the cerebellar gray matter (or maximal cerebellar activity) which spans the full thickness of the cortex. [2,3]. Variations in cerebellar activity, however, limit the usefulness of this attempt at absolute quantification for defining areas of abnormal perfusion. Another point to remember is that perfusion defects have been identified in up to 18% of asymptomatic control subjects [4]. Since there is an average of 11% variability between normal patients, it is difficult to establish a standard "normal" pattern of tracer uptake in the brain [5].
Patient preparation:
An I.V. line should be started 15-20 minutes before the tracer is administered. Patients should be injected with their eyes open and their ears unplugged (blood flow is increased by 30% in the occipital lobes when the eyes are open compared to closed). This should be done in a low light, reduced noise, and minimal traffic environment. Environmental conditions should be maintained for 10 minutes before and after the tracer is given. If the patients eyes are open in a bright room during injection, a higher tracer uptake in the calcarine cortex can be expected [8]. Caffeine containing products should be held for 24 hours prior to the exam. Imaging may be delayed for 1 to 11/2 hours after injection in order to improve clearance of blood pool activity.
Data Acquisition and Processing:
Because of the small size of cerebral structures, spatial resolution is a prime concern in brain imaging [8]. Most 3 headed camera systems used for CNS imaging are capable of spatial resolutions of 6 to 7 mm (full width at half maximum) in air. A high resolution collimator is recommended for CNS SPECT imaging [8]. Fan beam collimators which have converging collimation perpendicular to the axis of rotation can increase sensitivity by about 1.5 times. In general, the camera should come as close as possible to the patients head. A radius of rotation of 12 to 13 cm is desirable (if greater than 15-16 cm the images are probably not useful).
For image acquisition, the main goal is to achieve a balance between detection sensitivity (number of counts acquired) and the spatial resolution. These parameters are influenced by the dose of the agent used, the type of collimator employed, the pixel size, the number of projections, the acquisition time, and filtering [8]. Injecting the highest permitted dose will help to ensure adequate photon flux [8]. The matrix size chosen should provide a pixel size less than half the spatial resolution of the final image [8]. For example, if the final resolution of the brain SPECT slices is 10 mm, then the matrix size should be chosen to give a pixel size of 5 mm or less [8]. At a minimum, a 128 x 128 matrix should be used for image acquisition. Regarding the number of projections for the exam, it is best to keep the number close to the number of pixels in the matrix. For example, the best number of projections for a 128 x 128 matrix is one projection for each 3 degrees in a 360 degree orbit or 120 projections (for a 64 x 64 matrix, 60 projections [one each 6 degrees] would be optimal) [8]. A higher number of projections will yield minimal reconstruction benefits, and a lower number of reprojections will cause reconstruction artifacts [8]. Acquisition time is another factor to consider- longer acquisitions will yield a higher number of counts, but will also increase the risk for patient motion. During reconstruction, attenuation correction should be performed except when special devices that intrinsically correct attenuation, such a fan beam collimators of transmission attenuation correction systems are used [8]. Filtering of the data is another step to consider when reconstructing the data. Application of a smoothing filter will reduce the final resolution of the exam, thus, it would not be sensible to use a high resolution SPECT device and then lose information by applying a smoothing filter [8]. On the other hand, a very sharp filter will result in noisy images [8]. The number of final counts in the exam should be the guide for the best filter to apply [8]. Sharp filters can be used when the total number of counts is high, while lower count exams require a smoother filter [8]. It may be beneficial to have 3 or 4 predefined filters for different ranges of counts and apply these to the images to see which produces the best final image [8]. Images should be reformatted to a 15 degree axis to the cantomeatal line. This axis can be selected from a sagittal off center slice and orienting the images along a line drawn from the under surface of the frontal lobe to the inferior margin of the cerebellum. Temporal lobe long axis sections (transaxial slices parallel to the temporal lobe) are very useful in the evaluation of temporal lobe epilepsy. The decision to view the images in black-and-white or with a color display is arbitrary, however, a discontinuous multicolor scale may overestimate asymmetries [8]. Image normalization should be standardized. A good method is to use the maximum pixel count in the oblique slices [8].
Tc99m-HMPAO
(Hexamethylpropylenamine oxime): Ceretec?
Chemistry and Pharmacology
HMPAO is a lipophilic compound which is chemically unstable in-vitro (it undergoes oxidation). HMPAO exits in 2 isomeric forms: d,1-HMPAO (which has superior brain uptake and retention) and meso-HMPAO. Following IV administration, the agent is rapidly protein bound. It has a first pass extraction of about 80%. The distribution of the tracer is proportional to the regional cerebral blood flow, however, the ratio of gray to white matter activity is about 2.5:1 compared to the expected ratio of 4:1. Activity parallels cerebral blood flow up to 200 ml/min/100 gm of tissue (normal gray matter blood flow is about 80 ml/min/100 gm). HMPAO appears to overestimate low flow slightly, while underestimating areas of high flow.
The Tc99m-HMPAO crosses the intact blood brain barrier by passive
diffusion. There is
prompt CNS uptake with peak activity occurring within 1 to 2
minutes after injection with
between 4 to 7% of the injected dose remaining within the brain.
This initial prompt
uptake is followed by a rapid washout over 10-15 minutes of about
15% of the brain
activity. The activity which remains is then fixed in the brain
via conversion to a
hydrophilic compound by glutathione that cannot diffuse back out
of the cell
(intracellular oxidation of HMPAO by glutathione traps it inside
the neurons and glial
cells). Activity persists without washout for up to 24 hours.
Imaging is typically performed 30-90 minutes following tracer
injection [19].
HMPAO is highly unstable in vitro. A high radiochemical purity must be assured before injection as only a small portion of the injected dose will reach the brain [8]. Purity is not difficult to achieve if the timing of the labeling process is done properly. A fresh (no more than 2 hours old), uncontaminated eluate of Tc99m is necessary for satisfactory labeling. The generator should have been eluted in the preceding 24 hours. High eluate activity may reduce labeling efficiency. Due to rapid decomposition of the compound in vitro to a hydrophilic compound which will not cross the blood brain barrier, the agent must be used within 20-30 minutes of its preparation. A radiochemical purity of less than 85% or mixing the sample with blood in the syringe prior to injection results in poor image quality (the lipophilic agent will enter the RBC's). Stabilized forms of HMPAO using either methylene blue or cobalt chloride are available and allow easier labeling and improved image quality with reduced background activity [8].
Technetium Ethylene Cysteine Diethylester:
Tc99m-ECD: Neurolite?
Tc99m-ECD is a lipophilic cerebral blood flow agent that has moderate first pass cerebral extraction (60-70%) which results in an underestimation of regional cerebral blood flow (at high flow rates there is less uptake of ECD compared to the actual rCBF [11]). Brain uptake (due to lack of back diffusion) and blood clearance (due to renal excretion), however, is more rapid than that observed with Tc99m-HMPAO and this results in less extracerebral activity and a higher brain-to-background ratio. Peak activity occurs 1 to 2 minutes after injection and 6 to 7% of the injected dose is retained within the brain. Clearance from the brain parenchyma is very slow (about 6% per hour). The retention mechanism is related to rapid ester hydrolysis with conversion of the parent 1,1-isomer to a charged hydrophilic compound (tc-ethylcysteinate monomer [10]) that no longer has the capability of diffusing back across the blood brain barrier. There is rapid urinary excretion of the agent which results in a lower whole body radiation dose and which permits the administration of higher doses [8]. Unlike HMPAO, the compound is stable in-vitro (the agent is good for 4 to 6 hours after reconstitution, as compared to less than 30 minutes for Tc99m-HMPAO) and freshly eluted Tc-99m is not required for its preparation [8]. The labeling procedure is longer than for HMPAO, requiring about 30 minutes [8]. ECD image quality is slightly better than HMPAO due to the use of a high dose of the agent and a higher gray-to-white matter ratio [8].
Imaging is usually performed 30-60 minutes following tracer injection [19]. Studies have shown that there is some cerebral washout of the tracer during the first 2 hours following injection. This washout of activity from the brain may affect SPECT image reconstructions. It may be advisable to delay image acquisition for 2 hours, after which time washout is slower. Nonetheless, accurate diagnosis was not affected by early imaging in this study [6].
In subacute infarcts, during the period of luxury perfusion, ECD images can demonstrate a persistent defect due to low retention of the tracer in the area of infarction as a result of altered esterase function in hypoxia (hypometabolism) which results in an inability to fix the agent intracellularly [7]. In other words, Tc-ECD is considered to be a perfusion marker of viable brain tissue [10]. Tc-HMPAO fixation is not metabolically linked and therefore HMPAO imaging of subacute infarcts can demonstrate luxury perfusion which can result in an inability to properly identify areas of nonviable brain [8,10].
Comparison of the Pharmacokinetics of Tc-Radiopharmaceuticals for Perfusion Brain SPECT Imaging: [8]
| Parameter | Tc-HMPAO | Tc-ECD |
| Peak brain activity | 2 min | 2 min |
| Brain uptake (% injected dose) | 2-3% | 4-7% |
| Brain washout | 12-15% over 15 minutes | 12-14% the first hour; then 6%/hr |
| Excretion (% at 48 hrs after injection) | 50% liver-gut; 40% kidneys | 15% liver-gut; 75% kidneys |
| Target organs | Lacrimal glands; gallbladder wall | Gallbladder wall |
| Gray-to-white matter ratio | 2-3:1 | 4:1 |
| Imaging time after injection | Up to 4 hours | Up to 2 hours |
I-123 IMP (d, I-N-isopropyl-p-iodoamphetamine hydrochloride):
Dose is limited to 3-6 mCi.
The distribution of IMP reflects regional cerebral blood flow. IMP is lipophilic- it dissolves in the lipid membrane of the capillary vessels of the brain and rapidly passes through the blood-brain barrier by passive diffusion. Retention in the brain is controlled by binding to non-specific amphetamine binding sites on the brain cells. I-123 IMP binds to serotonin receptors and is dealkylated to iodoamphetamine. A subsequent oxidation of iodoamphetamine leads to a reactive product which covalently binds to microsomal proteins coupled with the mitochondrial P-450 [6].
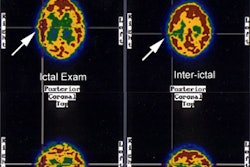
IMP has a high first pass extraction fraction of greater than 95% with a linear relationship between tissue activity and cerebral blood flow up to the high flow range. About 6 to 9% of the injected dose localizes to the brain. Peak brain activity is reached within 20 minutes. The remainder of the tracer predominantly localizes to the lungs (33%), liver (45%), and kidneys. Some delayed cerebral uptake occurs due to slow pulmonary release. Imaging must be done promptly as IMP metabolites will washout and redistribute over time. The optimal imaging time is about 20 minutes when the CNS levels of IMP reach a transient peak and cortical IMP uptake is approximately proportional to regional cerebral blood flow. Images obtained greater than 1 hour after injection show a loss of definition between cortex and white matter due to washout from the cortex and cerebellum which gradually fills in the white matter tracts. This latter distribution pattern more likely reflects amine binding sites. On I-123 imaging, areas of ischemia (i.e.: those with low CBF, but remaining metabolism) appear as defects on early images which "fill in" over time. Infarcted areas demonstrate decreased activity both on early and delayed images. In subacute strokes, during the period of luxury perfusion, in contrast to Tc99mHMPAO, IMP studies will still demonstrate a perfusion defect as the associated local acidosis decreases IMP uptake.
Xenon-133:
This agent can be administered by intra-arterial, intravenous, or by inhalation routes. Xenon is an inert, lipid soluble gas which does not undergo any chemical transformation in the brain. Regional cerebral blood flow (rCBF) and metabolism are generally directly related or coupled. The diffusable gas Xenon-133 is the best agent for determination of absolute quantitative blood flow (rCBF in mL/min/100 gm tissue) [8]. By measuring cerebral washout of the inhaled gas, an absolute rCBF can be obtained (washout is directly proportional to blood flow) [8]. Clinical use is unfortunately limited for several reasons. Xe-133's rapid transit and short biologic half-life in the brain, however, requires the use of high-sensitivity detector devices that are capable of acquiring rapidly changing image data in short time frames. Additionally, the low gamma energy of Xe-133 results in marked attenuation of deep structures which results in less than optimal SPECT images [8].
REFERENCES:
(1) Semin Nucl Med 1993; O'Tuama LA, Treves ST. Brain
single-photon emission computed
tomography for behavior disorders in children. 23: 255-64
(2) Nucl Med Annual 1993; Nagel JS, et al. Functional brain imaging in dementia. 29-45 (p.32)
(3) AJR 1994; Apr., p.944
(4) J Nucl Med 1994; Ichise M, et al. Technetium-99m-HMPAO SPECT, CT and MRI in the evaluation of patients with chronic traumatic brain injury: a correlation with neuropsychological performance. 35: 217-26
(5) Nucl Med Annual 1994; Mountz JM, et al. Brain SPECT: 1994 Update. 1-54 (p.8)
(6) J Nucl Med 1994; Moretti JL, et al. Early and delayed brain SPECT with technetium-99m-ECD and iodine-123-IMP in subacute strokes. 35: 1444-49
(7) J Nucl Med 1995; Moretti JL, et al. Cerebral perfusion imaging tracers for SPECT: which one to choose? 36: 359-363
(8) J Nucl Med 2001; Catafau AM. Brain SPECT in clinical practice. Part I: Perfusion. 42: 259-271
(9) J Nucl Med 2001; Camargo EE. Brain SPECT in Neurology and Psychiatry. 42: 611-623
(10) J Nucl Med 2001; Inoue Y, et al. Metabolism of 99mTc-ethylcysteinate dimer in infarcted brain tissue of rats. 42: 802-807
(11) J Nucl Med 1994; Tsuchida T, et al. SPECT images of technetium-99m-ethyl cysteinate dimer in cerebrovascular diseases: Comparison with other cerebral perfusion tracers and PET. 35: 27-31
(12) AJR 2004; Norfray JF, Provenzale JM. Alzheimer's disease: neuropathologic findings and recent advances in imaging. 182: 3-13