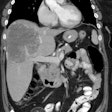
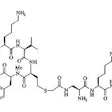
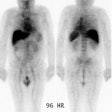
Technetium-99m Sestamibi Tumor Imaging:
Technetium 99m Sestamibi Tumor Imaging General Considerations:
The accumulation of Tc-Sestamibi (Tc-MIBI) in tumors is likely related to a number of variables. Tc-MIBI is a lipophilic monovalent cation (an isonitrile compound). It enters the cell via passive diffusion across plasma and mitochondrial membranes. It is postulated that Tc-MIBI accumulates within the mitochondria and cytoplasm of cells on the basis of electrical potentials generated across the membrane bilayers. At equilibrium it is sequestered largely within mitochondria by a large negative transmembrane potential. The agent is fixed intracellularly as long as cell membrane integrity is intact and nutrient blood flow persists.
Washout of Tc-MIBI from tumor cells is related to the energy (ATP) dependent transmembrane transporter proteins which include the P-glycoprotein pump system (P-gp) and the multidrug resistance protein (MRP) [7,8,38]. Tumor cells with a higher concentration of these transmembrane proteins demonstrate a faster rate of Tc-MIBI clearance (and hence, less tracer uptake) [7,8,22]. MIBI tumor washout can aid in identification of multi-drug resistant tumors and may provide prognostic information [38]. P-gp is also highly expressed at the lumenal endothelium of the human blood-brain barrier and this prevents entry of many CNS-active drugs [49]. P-gp inhibitors such as tariquidar have been developed as an adjunct for drug-resistant tumors and may also aid in increasing brain penetration of CNS medications [49].
There are many advantages to using Technetium rather than Thallium for scintigraphic imaging. Technetium's shorter physical half-life permits the use of a higher dose of the radiopharmaceutical [37]. This translates to a higher count rate which will shorten imaging times and provide sharper pictures [37]. The gamma energy of Technetium (140 keV) is optimal for use with the detector crystal used in the gamma camera and will undergo less attenuation and scatter. Technetium is also readily available and produced daily from a Molybdenum generator in most Nuclear Medicine departments. Since its introduction, Tc-99m-Sestamibi has been shown to be of value in the evaluation of many tumors.
Bone and Soft Tissue Tumors:
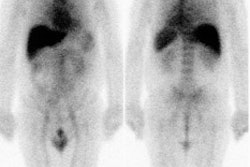
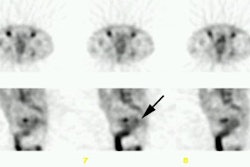
Tc-MIBI has been evaluated for distinguishing benign from malignant bone lesions [22]. Sensitivity has been reported to be 81%, and specificity 87%. Tc-MIBI may be particularly useful in evaluating sites of fracture- pathologic fractures demonstrate increased Tc-MIBI accumulation, while non-pathologic fractures do not [22]. False positive findings can be seen in myositis ossificans, osteoid osteomas, non-ossifying fibromas, and giant cell tumors [22].
Tc-MIBI has also been used in assessing malignant bone and soft tissue tumor response to therapy [28]. Like thallium, uptake is non-specific and can be seen in both benign and malignant lesions. Tc-MIBI permits the acquisition of flow images which are not possible with Thallium. [16]
Despite improved clinical outcome in osteosarcomas patients through the use of multiagent chemotherapy regimens, systemic relapses occur in about 50% of cases [38]. Conventional cytotoxic agents such as doxorubicin used in the treatment of osteosarcoma are substrates of multidrug resistance proteins which can limit the agents effectiveness [38]. Following Tc-MIBI injection, measurement of tumor to background activity at 10 and 60 minutes post injection can be used to determine the percent washout of the agent [38]. A higher washout indicates multidrug resistance protein expression by the tumor and a higher likelihood that the tumor will not have a complete response to therapy [38].
CNS Neoplasms:
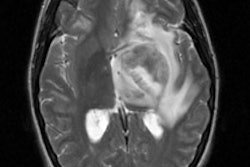
Tc-Sestamibi can be used to confirm CNS malignancy and can be particularly useful in differentiating neoplastic from non-neoplastic intracranial hemorrhage (ICH) [42]. Scans performed within 5 days of the event (and preferably within 2 days) will show tracer uptake in neoplastic ICH, but no tracer accumulation in nonneoplastic ICH [42]. If imaging is delayed, Tc-MIBI uptake can also be seen in non-neoplastic ICH- so early post-event imaging is critical [42]. Unfortunately, choroid plexus activity seen with Tc-sestamibi limits its usefulness for CNS neoplasm imaging [15] and choroid plexus uptake is not blocked by the use of perchlorate. In a comparison of Tc-MIBI with FDG PET for the detection of recurrent CNS neoplasm, Tc-MIBI was found to be of limited value [40].
Tc-Sestamibi has also been used to evaluate CNS neoplasms response to therapy. Tc-MIBI uptake is a marker of mitochondrial oxidative capacity. Tc-MIBI uptake correlates with the mitochondrial marker malate dehydrogenase. The addition of the mitochondrial uncoupler CCCP (which contains cyanide) can release 85% of the Tc-MIBI. In patients responding to chemotherapy, Tc-MIBI uptake within the lesion frequently decreases and this is felt to be reflective of damage to the mitochondrial oxidative capacity of the tumor.
Breast Cancer:
For the evaluation of focal breast lesions:
Mammography is the method of choice for the early detection of
clinically occult breast cancer [25]. A 30% reduction in mortality
has been reported among women enrolled in mammographic screening
programs [25]. Screening mammography has a relatively high
sensitivity of nearly 90%, but is limited by its lack of
specificity which is only about 35-54%, even in specialized
centers [23,25]. The utility of mammography is especially limited
in patients with large amounts of glandular tissue and dense
breasts [25].
Tc-sestamibi (Tc-MIBI) has been used to evaluate breast lesions.
Tc-sestamibi binds to mitochondrial cells and increases in
mitochondrial density typically denote cellular proliferation
[59]. Because cancer cells have higher proliferation, they will
demonstrate an increase in tracer uptake compared to the
surrounding tissue [59]. However, uptake and retention of tracer
in breast cancers appears to depend on several factors such as
regional blood flow, increased mitochondrial concentration in
cancer cells, increased angiogenesis, and tissue metabolism
[46,56]. Unlike mammography, the Tc-sestamibi examination is not
affected by breast density [6,32]. Background uptake of
Tc-sestamibi can be greater in the luteal phase of the menstrual
cycle and in postmenopausal women using hormone replacement
therapy and tends to be greater in dense breasts [54].
Breast specific gamma imaging systems have been developed and
have improved detection of small breast lesions compared to
conventional gamma cameras [55]. Dual head CZT solid state breast
imaging systems are the state of the art for molecular breast
imaging [55]. Previously, the typical dose was approximately 20-30
mCi of Tc-Sestamibi, but a dose as low as 7 to 10 mCi can be used
for high-resolution breast-specific CZT cameras [50,52,55].
Specially designed dual head CZT imaging systems with slight
breast compression have been developed to permit optimal imaging
of the breasts [52]. The dual head design permits increases
sensitivity (90% vs 80% with single detector system) and also
increases detection of sub-centimeter tumors compared to single
headed camera systems [52,53]. The increased sensitivity is due to
decreased distance between the detector and the lesion [53].
Standard CC and MLO projection images of each breast should be
acquired [55]. High resolution planar images are acquired for 7 to
10 minutes per view (or 100,000 counts [53]) [52,55]. The exam
lasts approximately 40-45 minutes [53].
Studies have demonstrated that if patients are in a fasting (3
hours), resting, and a warm state the uptake of Tc-sestamibi in
breast tissue is improved [55]. In pre-menopausal women, MIBI
imaging is best performed during the follicular phase of the
menstrual cycle, between days 2 and 12, when fibroglandular breast
tissue is not as physiologically active and should have lower
sestamibi accumulation [57,60]. Early imaging is more sensitive
than delayed studies as sestamibi accumulation within breast
lesions decreases significantly by one hour after tracer
administration [34,36]. Optimum imaging should begin within 5 to
10 minutes after tracer injection [36,55]. There may be adherence
of tracer in the regional veins after injection making evaluation
of the axilla and upper breast suboptimal- the arm opposite the
side of the lesion or the foot should therefore be injected.
High-resolution breast-specific gamma cameras provide a smaller
organ-to-detector distance and the ability to detect subcentimeter
lesions [50]. Lesions as small as 4 mm can be detected when using
breast specific, high-resolution gamma imaging systems [23,45].
When combined with conventional mammography the two exams have an
overall improved sensitivity for malignancy [21].
Overall, Tc-Sestamibi has a sensitivity of 70-96% and a
specificity of 60-100% for determination of breast malignancy
[6,19,21,23,25,31,32,34,51,59] (the higher sensitivities are
associated with the use of high-resolution breast-specific gamma
cameras [50]). The overall sensitivity is not affected by breast
density [50,51]. The reported sensitivities are similar to that
reported for FDG PET imaging (although FDG images usually
demonstrate a greater tumor to normal tissue activity ratio) [19].
The negative predictive value has been reported to be between
81-97%. A comprehensive review of the literature found a total
average sensitivity of 84.5%, average specificity of 89%, average
positive predictive value of 89%, average negative predictive
value of 84%, and an average accuracy of 86% [24]. Most false
negative exams occur with lesions smaller than 1 to 1.5 cm in size
or non-palpable lesions [21,32,35]. Studies indicate that the
exams sensitivity drops to 51% to 72% for non-palpable lesions
[2,3,6,20] (and the lower sensitivity is probably more accurate
[6]). A recent multicenter prospective trial [20] found an overall
institutional sensitivity of 75.4%, a specificity of 82%, a
positive predictive value of 74.5%, and a negative predictive
value of 83.4% (with a disease prevalence of 40%). In this same
study, the sensitivity for tumors under 1 cm in size was only
48.2% [20]. In another study, sensitivity for tumors less than 1
cm was 55.6% [51]. In the European multicenter study, the
sensitivity was 71% and the specificity was 69% [41].
Large lesions may also go undetected [21]. In one study, 19% of false-negative exams occurred in lesions over 3 cm in size [31]. Negative exams in large lesions may be related to: 1- overexpression of the multidrug resistance gene; 2- lesions with low desmoplastic activity or low cellular proliferation; and 3- lesions with low cell counts, low vascularity, and absence of inflammation [31,41]. Detection of smaller lesions and sensitivity is improved with the use of a high-resolution breast specific gamma camera, but the equipment costs make such a unit impractical for routine clinical use [35,44,47].
False positive exams have been described with lymph nodes,
fibroadenomas, papillomas, epithelial hyperplasia, mastitis, fat
necrosis, scleradenosis, and fibrocystic breast disease
[1,11,31,57]. Patients with fibrocystic disease are more likely to
have false-positive examinations [6]. It has been noted that high
resolution images of the breasts with either thallium or Tc-MIBI
may demonstrate some normal glandular activity, but this is
usually bilateral and non-localizing in character [1,2]. Washout
of tracer from both benign and malignant lesions is variable and
does not aid in lesion differentiation [6]. Quantification of
uptake has also not been of value in differentiating benign from
malignant lesions [6]. Tc-MIBI may be superior to Tc-tetrofosmin
for the evaluation of breast malignancies based upon in vitro
studies [17]. SPECT images provided better lesion contrast, but
were more difficult in determining lesion localization [4]. Also,
SPECT images often bring out the non-homogeneous characteristics
of patients with fibroglandular breasts resulting in an increased
risk for a false-positive interpretation [6].
Molecular breast imaging has also been studied as an adjunct to
mammography in patients with dense breasts [52]. In one study of
patients patients with dense breasts, 14 patients had cancer
detected by MIBI imaging alone [52]. However, the addition of MIBI
imaging increased the recall rate from 11% to almost 18% and the
biopsy rate from 1.3% to 4.2% and two small cancers (less than 5
mm) were not detected scinitgraphically [52]. In patients with
newly diagnosed breast cancer, it has been reported that MIBI
imaging can detect additional foci of occult breast cancer in 9%
of women [59].
It is generally accepted that Tc-MIBI is not accurate in the detection of malignant axillary adenopathy (sensitivity 38-60%) [1,2,19], although sensitivities as high as 79% to 84% have been reported [5,10].
Sestamibi imaging has also been used to monitor response to
therapy [47,60]. Residual uptake after therapy is indicative of
residual disease [47]. In one study, Sestamibi had a sensitivity
of 70% and a specificity of 90% for determining complete response
after neoadjuvant chemotherapy (compared to 83% and 60% for MRI,
respectively) [60]. Sensitivity of sestamibi was affected by tumor
size, with a sensitivity of 60% for tumors 1 cm or less in
size (compared to 77% for MRI) [60].
Radiation dose:
The estimated whole body effective dose is 5 mSv using 600 MBq
(16 mCi) of Tc-sestamibi [58] and 8 mSv for a dose of 25 mCi [60].
Using standard doses, the radiation dose is estimated to be 10-20
times higher than that of mammography [53].
However, using a low dose (8 mCi or 296 MBq) exam with a
dedicated dual head CZT camera system, the patient's effective
dose is decreased to 2 to 2.4 mSv [52,61]. Sestamibi has a
propensity to adhere to the plastic walls of syringes, thus
decreasing the administered dose by 20-30% and it has been
suggested that the actual administered dose is about 6.5 mCi for
an effective patient radiation dose of 1.9 mSv [61]. The dose may
be further reduced to 4 mCi (148 MBq) for an effective radiation
dose of less than 1 mSv [61].
The average effective dose from digital mammography is about 0.5
mSv and the effective dose from mammography with tomosynthesis is
1.2 mSv [52,58], so the MIBI examination dose is approximately
twice the dose of a standard 2D mammo with tomosynthesis [61].
However, although the whole-body radiation dose is estimated to be
2 mSv from the MIBI exam (and 0.5 mSv for standard mammography),
the actual radiation dose to the breast is orders of magnitude
lower than mammography (MIBI is distributed throughout the body,
whereas mammo x-rays are directed at the breast [61].
Words of caution: Tc-MIBI is not competitive with
mammography on either a cost effective or sensitivity basis for
screening patients [6]. All articles regarding Tc-MIBI in the
evaluation of breast masses suffer from 2 major drawbacks- 1- The
reported results for these studies focuses on a preselected
patient population- as a result the incidence of cancer in the
patients sent for the exam is usually very high 32-100%. This
suggests a selection bias and sensitivity of the exam is likely
overestimated [9]. 2- The mean lesion size is generally over 1.0
to 1.5 cm- by this size, a lesion will usually have fairly
characteristic mammographic or sonographic findings which can aid
in differentiating a benign from a malignant lesion [11].
Furthermore, the cancer detection rate with MIBI is lower than the
rate reported for breast MRI in average-risk women with dense
breasts (8.8 per 1000 woman screened compared to 15.5 per 1000
woman screened by MRI) ][61]. Other drawbacks include the lack of
an adequately high negative predictive value which means malignant
lesions may be missed [6,11] and false positive exams occur in
benign lesions such as fibroadenomas and inflammatory conditions.
Biopsy remains the most accurate way to determine whether a
lesion is benign or malignant. Stereotactic and ultrasound guided
core biopsies of breast lesions are minimally invasive and have a
high yield to provide a definite diagnosis. Fine needle biopsies
are known to often yield inconclusive results and it is
inappropriate to use FNA results as an end point for evaluating
the usefulness of scintimammography [43]. Despite optimism in the
nuclear medicine literature [43], this exam probably has only a
minor role in selected cases for the evaluation of patients with
suspected breast malignancy [18]- possibly in patients with
palpable masses, but no mammographically detectable abnormality
due to dense breasts [31]. However, ultrasound or MRI would still
be a better exam to initially evaluate these patients.
Unfortunately, almost all of the Tc-MIBI studies have not
adequately incorporated breast ultrasound or MRI into the patient
management scheme. In fact, contrast enhanced dynamic MR imaging
has been shown to have a higher sensitivity than Tc-MIBI - 96% vs
80% [25].
One recent retrospective study focusing on a small number of patients with biopsy proven invasive lobular carcinoma (i.e.: 100% of patients had biopsy proven invasive lobular carcinoma), suggested that the sensitivity of Tc-MIBI (93%) performed on a breast specific gamma camera, was higher than mammography (79%), sonography (68%), and MRI (83%) [45]. Despite the apparent better sensitivity, statistical analysis did not demonstrate a significant difference in detection of invasive lobular carcinoma between the modalities due to the small number of patients included in the study [45]. Also- there is the issue of which technique should be used to biopsy lesions detected only on gamma imaging [45]. None-the-less, there may be a role for gamma imaging in the evaluation of invasive lobular carcinoma- however, larger multicenter, prospective studies will be required to confirm this [45].
For the evaluation of multidrug resistance:
Tumor resistance to chemotherapy is in part mediated through an over-expression of the P-glycoprotein pump and other associated multidrug resistant glycoproteins which are the product of the multidrug resistance gene MDR1 [39]. These glycoproteins are responsible for the outward cellular transport of a variety of chemotherapeutic agents (such an daunorubicin, vincristine, and adriamycin) [33,39]. 99mTc-sestamibi is a substrate for the P-glycoprotein pump and a correlation exists between the efflux rate of 99mTc-sestamibi and the expression of P-glycoprotein in breast cancer [33,39]. By performing early and delayed 99mTc-sestamibi imaging of patients with breast cancer the washout rate of sestamibi can be determined. Lack of significant tracer washout indicates a low risk for chemoresistance [33]. On the other hand, low tracer accumulation and a high washout rate are associated with a high probability for chemoresistance (sensitivity 100%, specificity 80%, and positive predictive value 83%) [33,48]. In these patients, the use of chemo-revertant or chemomodulator agents could be justified [33].
Thyroid Cancer:
Tc-MIBI has also been used in the evaluation of metastatic thyroid cancer. The overall sensitivity of Tc-sestamibi for the detection of thyroid cancer ranges from 36% to 89%, and the specificity is 89-100% [37]. Early imaging (10-30 minutes after tracer administration) will detect more lesions [37]. Sestamibi is particularly sensitive for the detection of nodal metastases [37]. The agent has poor sensitivty for the detection of lung metastases and residual neck bed thyroid tissue [37]. The agent is particularly useful for follow-up of high risk patients with elevated thyroglobulin and negative radioiodine scans, and in patients with hurthle cell or medullary carcinoma. [13,14,27,30]
REFERENCES:
1. J Nucl Med 1995; Maurer AH, et al. Limitations of
craniocaudal thallium-201 and technetium-99m-sestamibi
mammoscintigraphy. 36(9):1696-700
2. J Nucl Med 1995; Khalkhali I, et al. Technetium-99m-sestamibi scintimammography of breast lesions: clinical and pathological follow-up.36(10):1784-9
3. J Nucl Med 1996; Villanueva-Meyer J, et al. Mammoscintigraphy with technetium-99m-sestamibi in suspected breast cancer. 37(6):926-30
4. Radiology 1995; Khalkhali I, et al. Scintimammography: the complementary role of Tc-99m sestamibi prone breast imaging for the diagnosis of breast carcinoma. 196(2):421-6
5. J Nucl Med 1995; Taillefer R, et al. Technetium-99m-sestamibi prone scintimammography to detect primary breast cancer and axillary lymph node involvement. 36(10):1758-65.
6. Semin Nucl Med 1997; Waxman AD. The role of Tc-99m-methoxyisobutylisonitrile in imaging breast cancer. 27 (1): 40-54
7. J Nucl Med 1997; Vecchio SD, et al. Fractional retention of Tc-99m-Sestamibi as an index of P-glycoprotein expression in untreated breast cancer patients. 38: 1348-1351
8. J Nucl Med 1997; Kostakoglu L, et al. Clinical validation of the influence of P-glycoprotein on Tc-99m-sestamibi uptake in malignant tumors. 38: 1003-1008
9. J Nucl Med 1997; Tiling R, et al. Comparison of Technetium-99m-sestamibi scintimammography with contrast-enhanced MRI for diagnosis of breast lesions. 38: 58-62
10: J Nucl Med 1998; Taillefer R, et al. Metastatic axillary lymph node technetium-99m-MIBI imaging in primary breast cancer. 39: 459-464
11. J Nucl Med 1999; Prats E, et al. Mammography and Tc-99m-MIBI scintimammography in suspected breast cancer. 40: 296-301
12. J Nucl Med 1999; Khalkhali I, et al. Procedure guideline for breast scintigraphy. 40: 1233-1235
13. J Nucl Med 1998; Miyamoto S, et al. Evaluation of technetium-99m-MIBI scintigraphy in metastatic differentiated thyroid carcinoma. 38: 352-356
14. J Nucl Med 1999; Seabold JE, et al. Comparison of 99mTc-Methoxyisobutyl Isonitrile and 201Tl scintigraphy for the detection of residual thyroid cancer after 131I ablative therapy. 40: 1434-1440
15. Nucl Med Annual 1994; Mountz JM, et al. Brain SPECT: 1994 Update. 1-54 (p.51)
16. J Nucl Med 1997; Taki J, et al. Evaluating benign and malignant bone and soft tissue lesions with technetium-99m-MIBI scintigraphy. 38: 501-506
17. J Nucl Med 2000; Rodrigues M, et al. Uptake of 99mTc-MIBI and 99mTc-tetrofosmin into malignant versus nonmalignant breast cell lines. 41: 1495-1499
18. Radiol Clin North Am 2000; Pisano ED, et al. Digital mammography, sestamibi breast scintigraphy, and positron emission tomography breast imaging. 38: 861-869
19. J Comput Assist Tomogr 2000; Yutani K, et al. Comparison of FDG-PET with MIBI-SPECT in the detection of breast cancer and axillary lymph node metastasis. 24: 274-280
20. J Nucl Med 2000; Khalkhali I, et al. Diagnostic accuracy of 99m-Tc-Sestamibi breast imaging: Multicenter trial results. 41: 1973-1979
21. J Nucl Med 2001; Buscombe JR, et al. Prediction of the usefulness of combined mammography and scintimammography in suspected primary breast cancer using ROC curves. 42: 3-8
22. J Nucl Med 2001; Pinkas L, et al. 99mTc-MIBI scintigraphy in musculoskeletal tumors. 42: 33-37
23. Radiographics 2001; Polan RL, et al. Scintimammography in patients with minimal mammographic or clinical findings. 21: 641-655
24. Semin Nucl Med 1999; Taillefer R. The role of 99mTc-sestamibi and other conventional radiopharmaceuticals in breast cancer diagnosis. 29: 16-40
25. Eur J Nucl Med 2001; Imbriaco M, et al. Scintimammography with 99mTc-MIBI versus dynamic MRI for non-invasive characterization of breast masses. 28: 56-63
26. Cancer Research 1990; Delmon-Moingeon LI, et al. Uptake of the cation hexakis (2-methoxyisobutylisonitrile)-technetium-99m by human carcinoma cell lines in vitro. 50: 2198-2202
27. J Nucl Med 1992; Balon HR, et al. Technetium-99m-sestamibi uptake by recurrent hurthle cell carcinoma of the thyroid. 33: 1393-1395
28. J Nucl Med 1992; Caner B, et al. Technetium-99m-MIBI uptake in benign and malignant bone lesions: A comparative study with technetium-99m-MDP. 33: 319-324
29. J Nucl Med 1991; Caner B, et al. Increased accumulation of hexakis (2-methoxyisobutylisonitrile) technetium (I) in osteosarcoma and its metastatic lymph nodes. 32: 1977-1978
30. J Nucl Med 1991; O'Driscoll CM, et al. Localization of recurrent medullary thyroid carcinoma with technetium-99m-methoxyisobutylnitrile scintigraphy: A case report. 32: 2281-2283
31. J Nucl Med 2001; Alonso O, et al. Is 99mTc-sestamibi scintimammography complementary to conventional mammography for detecting breast cancer in patients with palpable masses? 42: 1614-1621
32. Radiology 2001; Khalkhali I, et al. 99mTc Sestamibi breast imaging for the examination of patients with dense and fatty breasts: multicenter study. 222: 149-155
33. J Nucl Med 2002; Sciuto R, et al. Prognostic value of 99mTc Sestamibi washout in predicting response to locally advanced breast cancer to neoadjuvant chemotherapy. 43: 745-751
34. J Nucl Med 2002; Massardo T, et al. Diagnostic value of 99mTc-methylene diphosphonate and 99mTc-pentavalent DMSA compared with 99mTc-sestamibi for palpable breast lesions. 43: 882-888
35. J Nucl Med 2002; Brem RF, et al. High-resolution scintimammography: a pilot study. 43: 909-915
36. J Nucl Med 2002; Piccolo S, Muto P. One step forward. 43: 916-917
37. Endocrine and Metabolism Clinics of North America 2001; Haugen BR, Lin EC. Isotope imaging for metastaic thyroid cancer. 30: 469-492
38. J Nucl Med 2003; Burak Z, et al. 99mTc-MIBI imaging as a predictor of therapy response in osteosarcoma compared with multidrug resistance-associated protein and p-glycoprotein expression. 44: 1394-1401
39. J Nucl Med 2003; Britz-Cunnignham SH, Adelstein SA. Molecular targeting with radionuclides: state of the science. 44: 1945-1961
40. J Nucl Med 2004; Henze M, et al. PET and SPECT for detection of tumor progression in irradiated low-grade astrocytoma: a receiver-operating-characteristics analysis. 45: 579-586
41. J Nucl Med 2004; Tiling R, et al. Tissue-specific effects on uptake of 99mTc-sestamibi by breast lesions: a targeted analysis of false scintigraphic diagnoses. 45: 1822-1828
(42) J Nucl Med 2005; Minutoli F, et al. Timing of examination affects reliability of 99mTc-methoxyisobutylisonitrile SPECT in distinguishing neoplastic from nonneoplastic brain hematomas. 46: 574-579
(43) J Nucl Med 2005; Mathieu I, et al. Inconclusive triple diagnosis in breast cancer imaging: is there a role for scintimammography? 46: 1574-1581
(44) Radiology 2008; Brem RF, et al. Breast-specific gamma imaging as an adjunct imaging modality for the diagnosis of breast cancer. 247: 651-657
(45) AJR 2009; Brem RF, et al. Invasive lobular carcinoma: detection with mammography, sonography, MRI, and breast specific gamma imaging. 192: 379-383
(46) J Nucl Med 2009; Lee JH, et al. The role of radiotracer imaging in the diagnosis and management of patients with breast cancer: part I- overview, detection, and staging. 50: 569-581
(47) J Nucl Med 2009; Lee JH, et al. The role of radiotracer imaging in the diagnosis and management of patients with breast cancer: part 2 - response to therapy, other indications, and future directions. 50: 738-748
(48) J Nucl Med 2009; Liu J, et al. Retention of the radiotracers 64Cu-ATSM and 64Cu-PTSM in human and murine tumors is influenced by MDR1 protein expression. 50: 1332-1339
(49) J Nucl Med 2009; Wagner CC, et al. A pilot study to assess
the efficacy of tariquidar to inhibit p-glycoprotein at the human
blood-brain barrier with (R)-11C-verapamil PET. 50:
1954-1961
(50) AJR 2014; Rechtman LR, et al. Breast-specific gamma imaging
for the detection of breast cancer in dense versus nondense
breasts. 202: 293-298
(51) AJR 2014; Park JY, et al. Breat-specific gamma imaging
correlations with mammographic and clinicopathologic
characteristics of breast cancer. 203: 223-228
(52) AJR 2015; Rhodes DJ, et al. Molecular breast imaging at
reduced radiation dose for supplemental screening in
mammographically dense breasts. 204: 241-251
(53) AJR 2015; Holbrook A, Newell MS. Alternative screening for
women with dense breast: breast-specific gamma imaging (molecular
breast imaging). 204: 252-256
(54) J Nucl Med 2016: Berg WA. Nuclear breast imaging: clinical
results and future directions. 57: 46S-52S
(55) AJR 2017; Hruska CB. Molecular breast imaging for screening
in dense breasts: state of the art and future directions. 208:
275-283
(56) AJR 2017; Rauch GM, et al. Multimodality imaging for
evaluating response to neoadjuvant chemotherapy in breast cancer.
208: 290-299
(57) Radiographics 2017; Shermis RB, et al. Molecular breast
imaging in breast cancer screening and problem solving. 37:
1309-1327
(58) AJR 2017; Collarino A, et al. First clinical experience
using stereotactic breast biopsy guided by 99mTc-sestamibi.
209: 1367-1373
(59) AJR 2018; Brem RF, et al. Gamma imaging-guided minimally
invasive breast biopsy: initial clinical experience. 210: 695-699
(60) AJR 2019; Kim S, et al. Breast-specific gamma imaging versus
MRI: comparing the diagnostic performance in assessing treatment
response after neoadjuvant chemotherapy in patients with breast
cancer. 212: 696-705
(61) AJR 2020; Dibble EH, et al. Molecular breast imaging in
clinical practice. 215: 277-284