Ann Surg 1997 Nov;226(5):621-31
Use of carcinoembryonic antigen radioimmunodetection and computed
tomography for predicting the resectability of recurrent colorectal cancer.
Hughes K, Pinsky CM, Petrelli NJ, Moffat FL, Patt YZ, Hammershaimb L, Goldenberg DM.
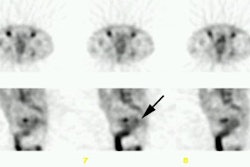
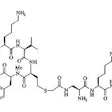
OBJECTIVE: The objective was to determine the role of arcitumomab (CEA-Scan; Immunomedics,
Morris Plains, NJ), an anticarcinoembryonic antigen (CEA) Fab' labeled with
technetium-99m, in the presurgical evaluation of patients with recurrent or metastatic
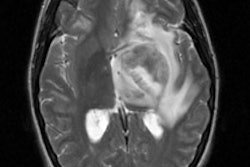
colorectal carcinoma. SUMMARY BACKGROUND DATA: Surgical resection is the only method known
to cure recurrent or metastatic colorectal carcinoma. The location and extent of disease
must be determined before surgery. The role of antibody imaging, a new cancer detection
modality, in preoperative evaluation for resection of locally recurrent or metastatic
colorectal cancer has not been established, either alone or in combination with standard
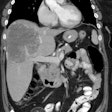
diagnostic modalities. METHODS: In a blinded analysis of 209 patients with known or
suspected colorectal cancer, the accuracy of arcitumomab, alone and combined with computed
tomography (CT), was compared to that of CT for predicting abdominopelvic tumor
resectability by correlating the results with surgical and histopathologic findings.
RESULTS: Arcitumomab alone or combined with CT was found to be significantly more accurate
for predicting surgical outcome than CT alone. When the results of CT and arcitumomab were
concordant for abdominopelvic resectability, nonresectability, or absence of disease, the
prediction was accurate in 67%, 100%, and 64%, respectively. Thus, the concordance for
nonresectability (100% correct) may obviate the need for other diagnostic modalities or
exploratory surgery. When the two tests were discordant, arcitumomab was correct
substantially more often than CT. Because the liver is the most common site of distant
metastasis in colorectal cancer, a subset of patients with hepatic disease was also
analyzed; findings were similar to the overall resectability results. The product's safety
profile was excellent: the incidence of induction of an immune response against
arcitumomab was <1% and that of potentially adverse events was 1.2%. CONCLUSIONS: The
accuracy of arcitumomab for assessing resectability status is greater than that of CT,
both in all patients undergoing evaluation for curative abdominopelvic resection of
colorectal cancer and in the subset of patients with suspected or proven liver metastases.
The additional use of arcitumomab with CT potentially doubles the number of patients who
could be saved the cost, morbidity, and mortality of unnecessary abdominopelvic surgery
and increases those who are potentially resectable for cure by 40%.