Magnetic tracers have emerged as a way to map ductal carcinoma in situ (DCIS) in patients, avoiding unnecessary sentinel lymph node biopsy and its inherent risks for patients. Researchers have touted its ease of use and cost-effectiveness.
"There's enough information already based on success for surgeons and patients to incorporate this into use today," said Dr. David Weintritt from Inova Health in Alexandria, VA. "It's very important when things like this come along to provide a service where nothing else can."
About 20% of patients with a preoperative DCIS diagnosis have invasive breast cancer on definitive histology, research suggests. This means sentinel lymph node dissection is needed.
 Magnetic tracing has been emerging for DCIS diagnosis as part of a procedure called "delayed" sentinel lymph node biopsy. Radiologists can inject the tracer in the area of interest to map nodes nearest to the DCIS, perform a mastectomy, and wait for analysis to confirm if it is invasive. Image courtesy of Endomag.
Magnetic tracing has been emerging for DCIS diagnosis as part of a procedure called "delayed" sentinel lymph node biopsy. Radiologists can inject the tracer in the area of interest to map nodes nearest to the DCIS, perform a mastectomy, and wait for analysis to confirm if it is invasive. Image courtesy of Endomag.While biopsy is unnecessary in most cases and could lead to lymphedema on rare occasions, radiologists have been challenged by how to accurately predict which women with a DCIS diagnosis will have invasive breast cancer.
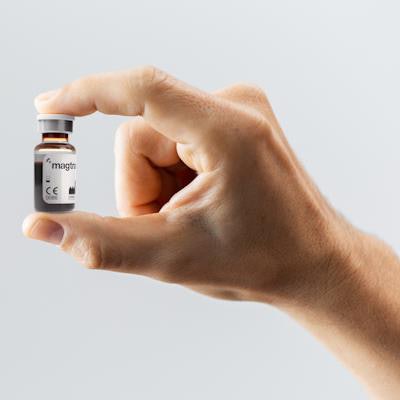
Through "delayed" sentinel lymph node biopsy, magnetic tracers can be injected in the area of interest by radiologists and remain in place for up to 30 days. Doctors can mark the nodes nearest to the DCIS, perform the mastectomy, and wait for pathologic analysis to confirm if it is invasive. Biopsy is performed only if the cancer is invasive.
The injection takes a few seconds and clinicians massage the area for several minutes to stimulate activity while the patient is under anesthesia. The tracer contains no radiation, and its core contains iron oxide.
"If 80% don't need a sentinel node biopsy, but you don't have a method of mapping someone after a mastectomy, then the beauty of the magnetic tracing technique is you get mapping during surgery and then make a decision about whether you need to remove the node up to three or four weeks later, maybe longer," Weintritt said.
Weintritt started performing breast surgery in the Inova system in 2003 and founded the National Breast Center in 2012 and National Breast Center Foundation in 2014 He began performing surgeries with magnetic tracing in 2020.
Recent research suggests this procedure's promise as well. A 2019 study led by Uppsala University Hospital found that 78.3% of patients avoided biopsy and that costs were reduced by an average of 24.5% in women without invasive breast cancer. Researchers are continuing to study this area in their trial known as Sentinot_2.
Another study presented in 2021 by University Hospitals Cleveland researchers demonstrated with a novel tracer that this procedure reduced surgical interventions in 87% of cases. The research was presented at the American Society of Breast Surgeons annual meeting.
However, Weintritt said experience and special instrumentation are needed to use this procedure. With the metallic component of magnetic tracing, there can "easily" be a false positive or difficulty in finding small lymph nodes. Also, patients who are allergic to dextran (used to coat the tracer's core) or iron oxide should not undergo this procedure, as well as those who may be prone to hemochromatosis.
"It [technique] requires understanding the parameters of what will and what will not result in success, and screening of patients to see who would meet those contraindications," Weintritt said.
Weintritt also said that while DCIS may not be viewed as life or death for patients, having techniques that prevent complications down the road are beneficial and important for patient quality of life.
"If you take this group and subject, five, eight, or 10% of them to a risk that becomes a lifetime impediment, it's really important to focus on techniques like this," he said. "You have a patient population that is predicted to survive their cancer, so it really is upon us to make sure that survival is combined with quality of life."