Cadens Imaging has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Cadens Colon virtual colonoscopy system.
Cadens Colon is designed to help radiologists review and classify suspicious regions, such as colonic lesions, according to Cadens.
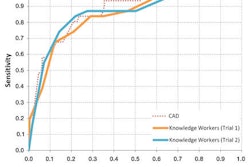
The technology features the company's proprietary tagging quality-tolerant electronic cleansing, along with an ergonomic user interface designed to enhance the evaluation, documentation, and follow-up of lesions.