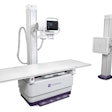
Konica Minolta Medical Imaging has received U.S. Food and Drug Administration (FDA) clearance for its AeroDR XE wireless digital radiography (DR) system.
With a weight of 5.7 lb, AeroDR XE is designed for use in extreme imaging environments such as emergency/trauma rooms and intensive care and critical care units, according to the vendor. It's also suitable for teaching hospitals, portable applications, and imaging at the bedside. AeroDR XE is resistant to bending and liquids and features a weight-to-load ratio up to 661 lb.
Konica Minolta said it has improved the detector's lithium ion capacitor, which now lasts a full shift or 300 images. The company has also incorporated new panel drop sensors and monitoring to provide ongoing data on panel handling. Technologists can also handle the panel using grip strips.