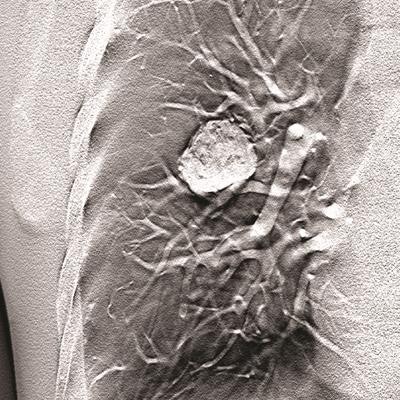
Carestream Health has garnered U.S. Food and Drug Administration (FDA) 510(k) clearance for its digital tomosynthesis (DT) software, adding a 3D capability to the firm's general radiography technology.
The tomosynthesis technology employs a single sweep of x-ray exposures and streamlines operator workflow by separating DT exposure acquisition from image volume formation, according to the vendor. This enables data to be generated from a series of low-dose x-ray images of an organ that were acquired at the same x-ray exposure but from different angles, Carestream said.
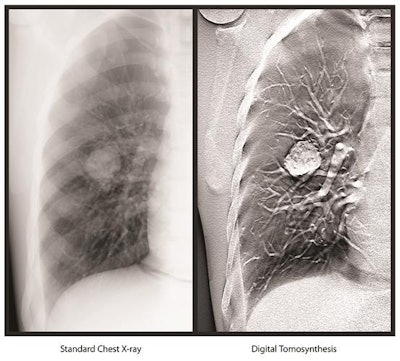 Image courtesy of Carestream.
Image courtesy of Carestream.The DT software is available as an upgradeable option on the vendor's DRX-Evolution Plus digital radiography system.



















