Samsung Electronics has received clearance from the U.S. Food and Drug Administration for GM85 Fit, a new digital radiography (DR) mobile x-ray system.
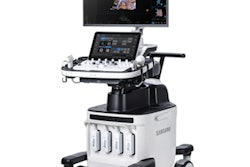
GM85 Fit is a new configuration of the company's premium GM85 mobile DR system and is designed to provide users with a more value-oriented option for mobile x-ray. The system includes a lead-free lithium-ion battery, and has features that support high efficiency, throughput, and efficient workflow, according to the company.
 Samsung's GM85 Fit mobile-x-ray system. Image courtesy of Boston Imaging.
Samsung's GM85 Fit mobile-x-ray system. Image courtesy of Boston Imaging.Differentiating features on GM85 Fit include full charging in three to four hours, ability to acquire up to 550 exposures and drive up to 37.3 miles during 11 hours of use without additional charging, and ability to operate at low noise levels during standby and Night Mode to avoid disturbing patients.
The system also sports an upgraded version of Samsung's S-Vue imaging processing engine, which is able to reduce halo artifacts around metallic objects and improve visualization of the bone-metal interface while reducing radiation dose levels. GM85 Fit also features the company's MirrorView function, which enables secure screen sharing for clinicians to review images, and tools like auto lung detection, S-Enhance, Sim Grid, and bone suppression.
GM85 Fit will be sold in the U.S. by Boston Imaging, the company's U.S. headquarters for sales of digital radiography and ultrasound products. Boston Imaging was formed earlier this year.