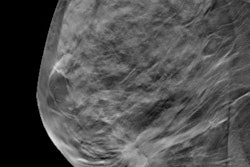
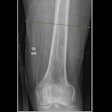
Israel-based Nano-X Imaging (Nanox) has received additional U.S. Food and Drug Administration (FDA) 510(k) clearance for the Nanox.ARC, an x-ray system incorporating advanced tomosynthesis technology to produce tomographic images for general use on adults.
These images may include the musculoskeletal system, pulmonary, intra-abdominal, and paranasal sinus indications.
The Nanox.ARC has a proprietary digital x-ray source. It utilizes advanced tomosynthesis technology with a cold cathode to create a more comprehensive sliced three-dimensional view of the body, enhancing visualization with multiple layers of images, and reducing the superimposition of structures that may be seen into dimensional x-rays, according to the company.
It has a smaller footprint than conventional stationary radiography systems, requires less power, and utilizes a matrix pattern that blurs out structural noise.