Computer-aided detection developer Rcadia Medical Imaging of Haifa, Israel, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for an enhanced version of its COR Analyzer software.
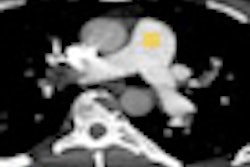
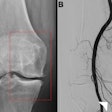
The expanded clearance supports the use of COR Analyzer to assess coronary branch vessels in addition to main coronary arteries in patients with suspected coronary artery disease. COR Analyzer automatically detects significant stenotic lesions (blockage of 50% or greater) in images generated by coronary CT angiography (CCTA) exams.