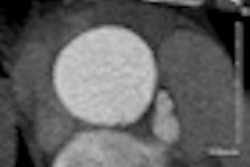
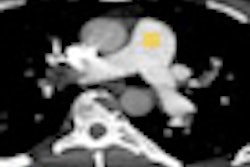
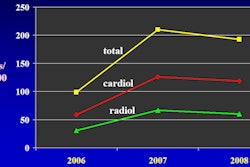
Interventional technology developer Medtronic said it will release U.S. clinical trial data on its Resolute drug-eluting stent at the upcoming annual scientific session of the American College of Cardiology (ACC) in April in New Orleans.
One-year results from the study, called Resolute US, will be presented in a late-breaking clinical trials session for interventional cardiology on April 4, according to Medtronic. The new data will complete Medtronic's submission to the U.S. Food and Drug Administration (FDA) for premarket approval of Resolute.
In addition, two-year results from the Resolute All Comers randomized, controlled trial will be highlighted in a featured clinical studies session on April 3. That trial compared Resolute with Abbott Laboratories' Xience V drug-eluting stent in a complex patient population representative of routine clinical practice, the company said.