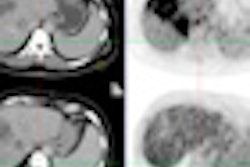
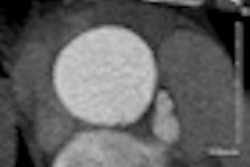
Radioembolization with an yttrium-90 (Y-90) radioisotope is a proven palliative treatment for liver metastases. And higher doses of Y-90 work even better, according to a study presented this week in Chicago at the Society of Interventional Radiology (SIR) annual meeting.
Results of a single-arm, prospective, multi-institutional study in 151 patients with liver metastasis demonstrated safety and tolerability of higher-dose treatment, according to Dr. Riad Salem, professor of radiology and director of interventional oncology at Northwestern University.
For these patients, who had no other options after chemotherapy had failed, the low-toxicity treatment consisting of two outpatient sessions preserved their quality of life. In the cohort, patients survived on average three to six months after treatment.
The average patient age was 63, and slightly more than half were male. All had unresectable tumors; 40% had developed them from colorectal cancers and 29% from neuroendocrine cancers. None of the patients had previously undergone Y-90 treatment. Prospective patients were excluded from the study if they had received external-beam radiation therapy, had severe liver or pulmonary dysfunction, and/or had contraindications to angiography.
The enrolled patients were treated from January 2007 through October 2009 at Albany Medical Center, Johns Hopkins University Health System, the Mayo Clinic in Rochester, the Medical College of Wisconsin, or Northwestern University. There were two treatment sessions, five weeks apart, after which patients were followed every 90 days for nine months, and every six months thereafter.
With this Y-90 radioembolization treatment, the microspheres (TheraSphere, Nordion) are injected through a catheter from the groin into the liver artery supplying the tumor. The beads become lodged within the tumor vessels, where they emit local radiation that causes cell death. This technique allowed for a higher local dose of radiation to be used (120 Gy ± 10%), with no danger to the healthy tissue, Salem explained. The median cumulative lung exposure, for example, was less than 10 Gy.
The primary acute toxicities reported within 90 days of treatment included fatigue (39%), nausea (28%), pain (18%), and anorexia (18%). There were 19 incidents of grade 3 toxicity and only two grade 4 incidents.
Patients with liver metastasis from colorectal cancer did not respond nearly as well as those whose primary cancer was neuroendocrine. Colorectal cancer patients had a median progression-free survival of 2.8 months and an overall survival of 9.4 months. Median progression-free and overall survival for neuroendocrine patients was 14.6 and 24 months, respectively.
The results were reproducible among all centers, and the study has set the stage for an international, multicenter phase III clinical trial, according to Salem.