Ten-year-old Katie Collman had never abused methamphetamine, but the drug may have killed her all the same. The resident of Crothersville, IN, is believed to have been kidnapped and murdered by methamphetamine dealers in her neighborhood earlier this year. Collman had stumbled upon a meth lab near her home and, in an effort to keep her quiet, her alleged abductors are accused of drowning the fourth grader in a nearby creek (CNN, February 7, 2005).
Clearly, the production of -- and demand for -- methamphetamine has grown so rabid that its traffickers are willing to take big risks to maintain their business and/or habit. But violent behavior is hardly unheard of when it comes to this substance, whose main ingredient was first discovered by Chinese doctors thousands of years ago as a way to stave off the common cold.
To this day, ephedrine -- the main ingredient in methamphetamine -- is used in over-the-counter cold and sinus remedies. In fact, meth makers rely on these meds by the case load to concoct their drug of choice, and lawmakers have taken note. Last month, a U.S. Senate bill was proposed that would place all ephedrine- and pseudoephedrine-based cold relief medications behind the pharmacy counter, and limit the number of pills purchased in a one-month period.
So what's the connection between meth and medical imaging? That would be in the brain, which sustains a "forest fire of damage," from meth abuse, according to one imaging expert. Neurology and mental health specialists are turning to MRI and PET to learn more about what draws people to meth, what keeps them on it, and how they might get off the drug (New York Times, July 20, 2004).
Meth madness
Meth also goes by the names speed, crank, or ice, which is its most lethal form. It can be smoked, snorted, or injected. Tweaking is a term of dubious distinction reserved for high-intensity users who spend from three to 15 days in a row on the drug.
Meth labs can spring up in your neighbor's garage or in an anonymous self-storage facility. Cooking crank usually involves ephedrine or pseudoephedrine, red phosphorous (used in the strike pad of matchbooks), and hydriodic acid (found in disinfectants). Lab explosions are common, as is asphyxiation by the phosphene gas that's produced if the brew is overheated. The crystal powder form comes about when the combustible liquid is married with a refrigerant.
 |
| A makeshift methamphetamine lab. Image courtesy of the California Attorney General's Crime and Violence Prevention Center and Stopdrugs.org. |
Although the stimulant has gained worldwide prominence, the U.S. is still meth central. In 2003, authorities dismantled more than 10,000 meth labs nationwide. California and Mexico jointly produce 80% of the world's meth, according to U.S. Drug Enforcement Administration (DEA) figures.
Meth closely resembles dopamine, artificially stimulating pleasure areas of the brain. During their high, users generally experience a general feeling of well-being coupled with extra bursts of energy.
Once they've come down, meth users can experience mild symptoms, such as irritability and loss of appetite, and more long-term issues such as kidney disorders and damage to the immune system. But it's the brain that takes the hardest beating.
Picking up speed
No one would dispute that meth is incredibly addictive. But precisely what process does the brain go through from that first puff to full-blown cravings? Mental health specialists from the U.K. tackled that question by tracing the reward circuitry activated by meth use.
Dr. Birgit Völlm and colleagues studied seven right-handed volunteers who were considered "psychostimulant-naïve," having no history of drug abuse. At the time of the study, Völlm was at the University of Oxford and Wameford Hospital in Oxford. Her co-authors included Drs. Peter Jezzard, Ronald Heal, and Paul Matthews from the Centre for Functional Magnetic Resonance Imaging of the Brain, John Radcliffe Hospital, also in Oxford.
The study protocol consisted of an intravenous (IV) injection of saline, followed by a one-minute shot of methamphetamine by IV (0.15 mg/kg), and more saline. The subjects were unaware of when the saline infusion was stopped and the drug infusion had begun. The volunteers then rated their "mind-racing" experience at one-minute intervals by pressing a button.
"We were interested in studying the effects of methamphetamine in reward-related areas when no motor-related responses were being given, to exclude the possibility that the activations observed resulted from an enhancement of central correlates of motor and related activity involved in making the ratings, including the button press, by amphetamine," they wrote (Neuropsychopharmacology, September 2004, Vol. 29:9, pp. 1715-1722).
Functional MRI (fMRI) was performed on a 3-tesla system (Inova, Varian Medical Systems, Palo Alto, CA). The authors used a set of optimizing techniques to highlight the orbitofrontal cortex, which is associated with reward and reinforcement. A disruption to the circuits in this area could lead to compulsive drug intake, they said. The data was analyzed using SPM99 software (Wellcome Department of Cognitive Neurology, University College London, U.K).
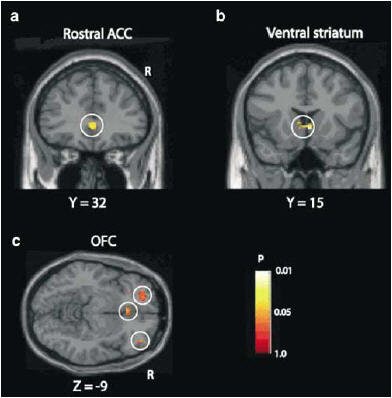 |
| Results of the SPM analysis correlating changes in BOLD signal with subjective ratings of "mind-racing" produced by methamphetamine. Correlated activations (shown within the white circles) were found in (a) the medial prefrontal cortex (rostral anterior cingulated cortex), (b) ventral striatum, and (c) orbitofrontal cortex. The p values shown are with the small-volume correction (SVC) procedure. Image courtesy of Dr. Birgit Völlm and Neuropsychopharmacology, September 2004, Vol. 29:9, pp. 1715-1722, figure 3, copyright 2004 by the Nature Publishing Group. |
The results showed that all the subjects reported a mind-racing experience within three minutes of meth infusion. On fMRI, there were significant activations in the medial part of the orbitofrontal cortex and in the rostral part of the anterior cingulate cortex. The fMRI signal and mind-racing ratings correlated in the caudate nucleus and in the rostral part of the anterior cingulate cortex.
The findings are significant in light of previous research showing a connection between the orbitofrontal cortex and drug addiction, the authors said. Because their subjects had no previous experience with psychostimulants, the results were not complicated by craving or cued recall, which are common in drug-dependent individuals.
The connection between addiction and the reward center is particularly important when dealing with adolescents at risk for meth abuse. Previous neuroimaging research has indicated that young people -- and their developing ventral striatums (VS) -- tend to be understimulated. As a result, adolescents may seek more extreme incentives as a way of compensating for low VS activity levels. Given the central role of the orbitofrontal cortex in reward and reinforcement, the developing brain might be that much more prone to addiction, Völlm concurred in an e-mail to AuntMinnie.com.
The next phase of research for Völlm's group will focus on what she called "pharmacoMRI," using serotonergic drugs, which are frequently given to meth abusers to stem depression.
"As well as looking at the direct effects of these drugs, we also will look at effects of the drugs on reward, loss, behavioral inhibition, and emotional recognition," stated Völlm, who is currently a clinical lecturer and specialist registrar in the forensic psychiatry, neuroscience, and psychiatry unit at the University of Manchester, U.K.
This is your brain on drugs
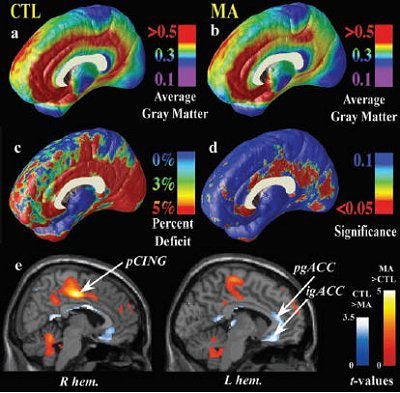
Given how quickly the reward center of the brain latches onto the high produced by meth, it's a short trip to long-term abuse. Researchers from the University of California, Los Angeles determined that consistent meth use can lead to physical changes in the brain, including an actual loss of brain matter. The group was lead by Paul Thompson, Ph.D., from the laboratory of neuroimaging at UCLA.
Thompson's group worked with 22 meth abusers with a history of dependence, as well as 21 people with no history of substance abuse, who served as the control group. The meth abusers had smoked the drug for 10.5 years on average, beginning in their mid-20s. They consumed around 3 g of meth per week and had used the drug during the month prior to the study.
All subjects underwent scans on a 3-tesla MRI scanner. Three-dimensional, T1-weighted, GRE structural scans were obtained and all images were processed with manual and automatic procedures. The imaging was overseen by co-author Edythe London, Ph.D., from UCLA's Brain Research Institute. The image processing involved tagging 20 standardized anatomical landmarks in each image set based on the International Consortium for Brain Mapping-305.
The images were analyzed for:
- Cortical pattern matching to localize disease effects on cortical anatomy
- Statistical maps to indicate differences in local gray-matter volumes
- Three-dimensional surface models to assess hippocampal atrophy
The authors found that in meth abusers "highly significant gray-matter deficit was observed in a broad anatomical region encompassing the cingulated gyrus, subgenual cortex, and paralimbic belts -- the most significant impairments occurred in cingulated regions, in which gray-matter volumes were 11.3% below the control average" (Journal of Neuroscience, June 30, 2004, Vol. 24:26, pp. 6028-6036).
They also found that hippocampal volumes were significantly smaller in meth abusers -- 7.8% lower on average. These subjects also performed poorly on word-recall tasks, with greater atrophy of the left hippocampus correlating with worse performance.
In comparison, there was notable hypertrophy of the white matter in the meth group, with a total white-matter volume checking in at 7% greater than controls. More specifically, there was 6.6% white-matter excess in the left hemisphere and 7.5% in the right.
"This expansion effect typically accompanies gray-matter atrophy, and is part of a structural pattern often found in neurodegenerative or psychotic disorders," such as Alzheimer's disease and schizophrenia, Thompson's group wrote.
"Chronic (meth) abuse is associated with a pattern of abnormal brain structure that is comparable with or greater than MRI deficits in early dementia and schizophrenia in terms of effect size," they stated.
Prenatal exposure
The effects of meth use in adults are crystal clear, but what about its impact on children exposed in utero? Learning about this abuse by proxy is particularly vital as more women are turning to the drug in an effort to expedite weight loss -- commonly known as the Jenny Crank Program. In the state of Utah, for example, meth was the most abused illicit drug among women seeking treatment (Utah Drug Threat Assessment, July 2003, Utah Division of Substance Abuse).
In a pilot study, Dr. Linda Chang and colleagues examined the effects of prenatal meth exposure on the developing fetal central nervous system, as well as on the structure of the brain. They studied brain morphometry on MRI of meth-exposed children and compared those findings to the brains of children who had not been exposed in utero.
"This preliminary study suggests children exposed to meth in utero have neurocognitive deficits and structural alterations despite normal appearing MRIs on routine clinical evaluations," wrote Change's group in Psychiatry Research: Neuroimaging (December 15, 2004, Vol. 132:2, pp. 95-106).
Chang is currently at the John A. Burns School of Medicine at the University of Hawaii in Honolulu. At the time this study was conducted, she was with the David Geffen School of Medicine at UCLA. Her co-authors are from UCLA and the Harbor-UCLA Medical Center in Torrance.
The study participants were 13 children (mean age 6.9 years) who had been exposed to meth during at least two-thirds of their time in utero. The control group consisted of 15 children with no history of meth exposure (mean age 7.8 years).
Only one meth-exposed child required sedation with chloral hydrate. MRI studies were performed on a 1.5-tesla scanner (Sigma 5.8, GE Healthcare, Waukesha, WI), starting with a sagittal, T1-weighted localizing image. Next, an axial fast inversion recovery scan was done, and an in-house automated program was used to segment the brain from the surrounding structures.
Finally, an inversion recovery sequence offered contiguous brain slices, as well as contrast between gray, white matter, and cerebrospinal fluid. These latter images were used for morphometric analyses. The regions of interest included the putamen, globus pallidus, and hippocampus. The scans were all read by co-author Dr. Irwin Walot, a neuroradiologist.
According to the results, "the meth-exposed children showed smaller volumes across all four subcortical gray-matter volumes and independent of hemisphere.... Significant meth effects on the relative volume were found in the putamen and in the globus pallidus.... In addition, meth-exposed children had smaller hippocampal volumes," the authors stated.
They also found a relationship between brain volume and performance on neurocognitive tests. They discovered that a smaller putamen and globus pallidus was related to poor performance on delayed verbal memory tests. In addition, poorer visual motor integration was associated with a smaller globus pallidus.
"The significant correlations between the attention and delayed memory problem and the smaller putamen and hippocampus would be consistent with deficits in the dopaminergic system, which are likely due to the prenatal meth exposure," they concluded.
Whether these changes in brain volumes persist into adulthood remains unclear. However, other studies have indicated that meth-exposed children do exhibit more aggressive behavior, as well as difficulties with language, math, and physical activities.
While the children in this study were not followed up, Chang told AuntMinnie.com that she and her colleague in Hawaii, pediatrician Dr. Chris Derauf, plan to perform a larger validation study with more refined MRI techniques. Derauf has already enrolled more than 100 meth-exposed patients in a separate behavioral study.
"Methamphetamine is ... a huge drug problem in Hawaii. (We) are planning to perform imaging (using MRI and MR spectroscopy) in a large group of infants exposed to methamphetamine," Chang wrote in an e-mail. "In this new study, we will use an MPRAGE sequence with 1.4-mm slice thickness," compared to the 3.5-mm slice thickness used in the previous study.
Kicking the habit
One of the conclusions of Thompson's study was that the MR revelations about structural abnormalities in meth users, as well as previous research on metabolic abnormalities, "suggest a substrate for cognitive disorder that could interfere with treatment of (meth) dependence."
Thompson's study builds on previous research done by his co-author, Edythe London, Ph.D. In this experiment, FDG-PET imaging was performed to judge mood disturbances and regional cerebral metabolic abnormalities in recently abstinent meth abusers.
"Stimulant abusers often enter treatment within their first week of abstinence," the group wrote. "Because treatment for (meth) abuse almost exclusively involves outpatient methods, the first week of abstinence is a crucial determinant of engagement and retention and, thus, of treatment outcome" (Archives of General Psychiatry, January 2004, Vol. 61:1, pp. 73-84).
For this study, brain imaging data was collected from 17 meth abusers and 18 control subjects. The former group had used the drug for more than eight years, consuming about 2-4 grams per week. They had used the drug for most of the 30 days before entering the study, but had been off meth for four to seven days at the time of the study.
Structural MR images were acquired first and then coregistered with the PET data (ECAT EXACT HR+, Siemens Medical Solutions, Malvern, PA; CTI Molecular Imaging, Knoxville, TN). PET scanning was performed in 3D mode, after the IV administration of ≤ 5 mCi FDG. The latter was given while the subjects underwent a continuous performance task.
Group comparisons of brain activity were done with SPM99 software. The analysis looked at relative regional cerebral glucose metabolism (rCMRglc). The regions of interest included the orbitofrontal cortex, cingulated gyrus, and amygdala. Activities in these areas, as well as others, have been linked to depressive disorders, depressed mood, and sadness, the authors explained. They correlated metabolism with depressive symptoms.
"The (meth) abusers had higher (depressive symptoms) scores than control subjects.... The abusers also had higher scores of both state and trait anxiety.... The abusers reported drug cravings (which) was correlated with frequency of use," the authors explained in their results.
Based on the imaging results, the meth group and the control group differed significantly in relative regional glucose metabolism, with the abusers showing lower rCMRglc in the anterior cingulate and insula. The rCMRglc was higher in several regions, including the lateral orbitofrontal area, the cerebellum, and the amygdala.
The authors reported a direct covariance between the depressive symptoms score and the relative rCMRglc in the amygdala in abusers, suggesting that amygdalar dysfunction contributes to depressed mood in this group. Finally, abnormalities observed in the limbic and paralimbic regions were noteworthy because those areas are associated with attention and conflict resolution.
"The findings identified brain substrates of affective dysregulation as potential targets for therapeutic intervention during early abstinence and withdrawal in (meth) abusers," London's group concluded.
Quitting crank
The next step would be to use these results to ramp up treatment protocols. Currently, there are no pharmaceutical treatments that are particularly effective in users; most recovery programs rely on therapeutic cognitive behavioral interventions. Antidepressants and selective serotonin reuptake inhibitors (SSRIs) are administered, although Völlm questioned their value.
"The rationale for administering antidepressants is not entirely clear to me. They might treat underlying psychological problems, which might indirectly cause drug use.... As far as SSRIs are concerned, they might help with impulsivity, i.e., impulsive drug-taking," she stated.
Imaging could also play a role in determining if some people are more neurologically prone to addiction. Völlm pointed out that dopaminergic systems tend to be underactive in meth users, so perhaps that's partly why they seek an artificial boost to their system.
"Imaging could help to find (a legal) drug, which activates similar systems (as) the addictive drugs," she suggested.
This physical propensity for substance abuse may also be notable in children with prenatal exposure to meth, although the neurological changes seen on MRI require a sophisticated eye, Chang said.
"The changes that we see are typically not easily detectable by a radiologist," she said. "The smaller volumes are visible only with careful measurements. Until we have some kind of automated quantitative measurements, with reference normal values, it would not be useful to just get an MR exam."
However, MRI did indicate that the situation is not completely hopeless for meth users: Thompson's group found that while the white matter in the addicts was inflamed, the actual structure of the connective fibers remained intact. Another research group from Brookhaven National Laboratory in Upton, NY, determined that former meth abusers showed improved glucose metabolism in the thalamus after staying off the drug for at least a year (American Journal of Psychiatry, February 2004, Vol. 161:2, pp. 242-248).
So even for the most out-of-control tweakers, staying off meth could mean reclaiming their brains, as well as their lives.
By Shalmali PalAuntMinnie.com staff writer
February 28, 2005
Front image courtesy of www.methamphetamines.org. Top image courtesy of McClatchy Company's California Newspapers.
Related Reading
Ex-meth abusers show some reversal in brain damage, March 15, 2004
Teenage wasteland? fMRI study shows adolescent brain is short on motivation, March 19, 2004
MRI, MRS piece together neurological damage in chemical solvent abusers, November 20, 2003
Cocaine abuse may irreversibly damage dopamine neurons, January 20, 2003
Copyright © 2005 AuntMinnie.com