Researchers in Massachusetts are showing progress in the development and application of a new PET tracer that could enhance the view of atherosclerotic inflammation.
Inflammation is the major catalyst underlying atherosclerosis, plaque rupture, and subsequent thrombosis, while monocytes and macrophages also have significant influence on a cellular level in lesion severity.
A monocyte will be "recruited into the lesion by adhesion molecules," explained Dr. Matthias Nahrendorf of the Center for Molecular Imaging Research at Massachusetts General Hospital (MGH) in Boston. "This process leads to more inflamed plaque, more vulnerable plaque, and a plaque that is more rupture-prone. Therefore, it is very interesting to image the macrophage content."
Having previously developed a dextran-coated magnetic nanoparticle that provides contrast in T2-weighted MR images and a carbohydrate shell to attach a fluorochrome to render the nanoparticle as an optical imaging agent, researchers at MGH and the Harvard Medical School in Boston sought to expand the cellular specificity of the nanoparticle to other imaging modalities.
The nanoparticle has been used previously in experimental and clinical MRI of the lymphatic system and to image inflammation. Given PET's greater sensitivity compared to MRI, hybrid PET/CT systems may "overcome a potential limitation when we look into vascular imaging using PET, because you can use the CT for inflammation to see where the signal comes from," Nahrendorf said.
Hence, the researchers developed the PET-detectable copper-64-based isotope, Cu-64 DTPA magnetofluorescent nanoparticle (CLIO-CU64-VT680).
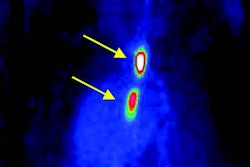
Peak PET activity
Specifically, the study investigated the value of the new nanoparticle and PET tracer for imaging inflammatory atherosclerosis using five mice. Iodine contrast-enhanced PET/CT imaging was performed 24 hours after injection of CLIO-CU64-VT680 during isoflurane anesthesia. Following PET/CT, the aortas were analyzed by scintillation counting and phosphor imager.
Fluorescence microscopy and immunoreactive staining was subsequently performed. The study also determined the blood half-life by repetitive retro-orbital bleeding and biodistribution in the mice. The half-life of CLIO-CU64-VT680 was 259 minutes. Autoradiography was used to corroborate in vivo findings.
The researchers found that peak PET activity mapped to areas of high plaque load identified by CT, such as the aortic root and arch. The imaging findings also correlated with fluorescence microscopy, which demonstrated preferential uptake of CLIO-CU64-VT680 into monocytes and macrophages and lower uptake in surrounding smooth muscle and endothelial cells.
The group also compared the uptake of the trimodality nanoparticle with that of the radiotracer FDG, which is used increasingly to image inflammatory atherosclerosis.
"It is an intriguing concept that you can pin down where you have increased metabolism by using this radioactive (material), which gets entrapped in cells with high metabolism," Nahrendorf said at the first annual joint conference of the Academy of Molecular Imaging (AMI) and Society of Molecular Imaging (SMI) in Providence, RI. "However, it is not easy to validate, and there are reports that correlate increased FDG activity with high macrophage content."
Better than FDG
In the comparison, the researchers found that the nanoparticles enhance aortic regions, while the uptake was lower in FDG.
The new PET tracer, based on the nanoparticle, "gives us robust in vivo PET signal in lesion-prone areas," Nahrendorf noted. "Autoradiography established that this activity comes from atherosclerotic plaques. The primary cellular targets are macrophages, and the trimodality character of this nanoparticle allowed us to perform rigorous validation."
In addition, Nahrendorf foresees that the nanoparticle will be beneficial for future hybrid imaging modalities, such PET/MRI. It also proved very sensitive, despite a low dose, as researchers were able to image plaques in the aorta, which is smaller in size than a coronary artery.
Because of the nanoparticle's versatility, ligands can be attached to it to target specific receptors or surface molecules, he added.
Nahrendorf also cited maximized sensitivity, potential whole-body imaging, quantification of the PET signal, and spatial localization of trireporter by microscopy as benefits of the new tracer.
By Wayne Forrest
AuntMinnie.com staff writer
November 15, 2007
Related Reading
Calcium contrast boosts T2-weighted signal in molecular MRI, December 6, 2006
SUNY Buffalo developing multimodality nanoparticles, October 6, 2006
Imaging's big future gets smaller, July 3, 2006
Image guidance evolves into imaging therapy, May 12, 2006
A new use for SPECT/CT: Tracking stem cell migration, October 7, 2005
Copyright © 2007 AuntMinnie.com