GE Healthcare said that final results from a phase III study of its F-18 flutemetamol PET imaging agent showed strong concordance between F-18 flutemetamol PET images and beta-amyloid brain histopathology.
GE believes that the study, which was presented this week at the Alzheimer's Association International Conference in Vancouver, British Columbia, confirms the potential of F-18 flutemetamol as an imaging agent to detect beta-amyloid plaque in living patients.
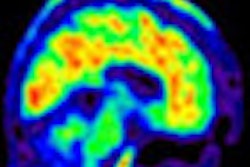
Presented by Dr. Milos Ikonomovic of the University of Pittsburgh Medical Center, the phase III study examined the autopsied brains of 68 subjects who had received F-18 flutemetamol imaging while living to detect the presence of brain beta-amyloid pathology. The images were then evaluated visually by independent trained readers, and flutemetamol retention in the brain was quantified using a standardized uptake value ratio.
The brains of subjects who subsequently died were then evaluated histopathologically and compared with interpretations of flutemetamol PET scans. Flutemetamol detected beta amyloid with a majority-read sensitivity of 86% and a specificity of 92%, according to the researchers.