PET scans comparing the location and magnitude of tau protein accumulation have shown that tau better predicts future brain atrophy in patients with Alzheimer's disease compared with amyloid deposition -- and a year or more in advance, according to a study published online January 1 in Science Translational Medicine.
Researchers from the University of California, San Francisco (UCSF) Memory and Aging Center found that tau buildup, as seen on baseline PET scans, predicted subsequent atrophy in the same locations with more than 40% accuracy, compared with 3% accuracy for amyloid in projecting brain degeneration.
"The match between the spread of tau and what happened to the brain in the following year was really striking," said senior author Dr. Gil Rabinovici, distinguished professor of neurology at the UCSF Memory and Aging Center, in a statement. "Tau PET imaging predicted not only how much atrophy we would see, but also where it would happen. These predictions were much more powerful than anything we've been able to do with other imaging tools and add to evidence that tau is a major driver of the disease."
The researchers enrolled 32 patients (mean age 64 ± 9 years; range, 49-83 years; 20 patients [63%] were less than 65 years) who were in the early clinical stages of Alzheimer's and who underwent a positive PET scan with carbon 11-labeled Pittsburgh Compound B (PiB), indicating the presence of amyloid protein, another biomarker for dementia. All of the subjects also underwent a structural 3-tesla MRI scan and PET imaging with flortaucipir (Avid Radiopharmaceuticals) to determine levels of tau protein. A second structural MRI scan was performed a median of 15 months later.
PiB-PET images revealed amyloid deposition in the prefrontal, posterior cingulate, and precuneus regions, as well as the lateral frontal and temporoparietal cortices. Flortaucipir-PET discovered maximum tau accumulation in the temporoparietal junction, the posterior cingulate, and the precuneus and moderate amounts in the dorsal frontal, occipital, and inferomedial temporal cortices.
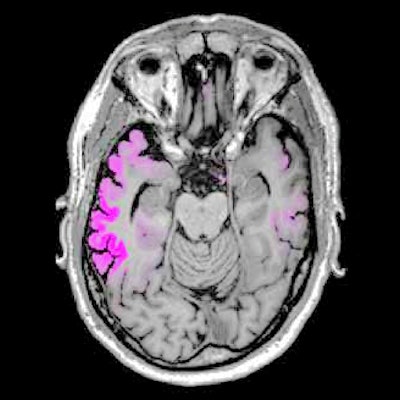
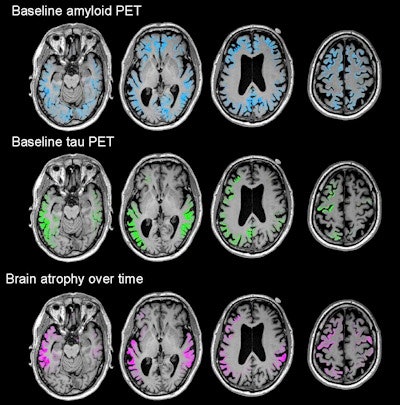 Images show four sequential cross sections of a single study participant's brain (left to right = bottom to top) to illustrate that the relationship between amyloid, tau, and future brain degeneration is similar across the brain, not just in the single cross sections shown in the other figures. Images courtesy of UCSF and Rabinovici et al.
Images show four sequential cross sections of a single study participant's brain (left to right = bottom to top) to illustrate that the relationship between amyloid, tau, and future brain degeneration is similar across the brain, not just in the single cross sections shown in the other figures. Images courtesy of UCSF and Rabinovici et al.The researchers longitudinally charted a pattern of atrophy that was greatest in the temporoparietal, posterior cingulate, precuneus, and dorsal frontal areas. All of those areas of atrophy coincidentally matched significantly elevated levels of flortaucipir.
Most importantly, those areas of tau buildup predicted subsequent atrophy in the same locations with more than 40% accuracy. Baseline PiB-PET scans, which detected amyloid, correctly predicted only 3% of future brain degeneration.
"The association between baseline flortaucipir-PET and subsequent atrophy, and notably the topographical similarity between the two patterns, was a strong and robust finding," Rabinovici and colleagues wrote.
At the same time, the researchers emphasized that their findings are not meant to contradict the role that amyloid plays in a person's possible progression to Alzheimer's disease.
"Our findings suggest that tau-PET could be useful for the design of clinical trials and could increase the ability to detect a treatment effect even over a relatively short time frame," they added. "First, tau-PET could be used to enrich trials with patients with tau-PET signal predictive of upcoming atrophy or to stratify patients in trials based on the degree of expected atrophy in the upcoming year. Second, tau-PET could help determine how (i.e., where) atrophy should be measured to maximize study sensitivity."