In what the agency called a regulatory first, the U.S. Food and Drug Administration (FDA) has approved the radiopharmaceutical flortaucipir F-18 for imaging tau pathology on PET scans. The agent was developed by Eli Lilly subsidiary Avid Radiopharmaceuticals and will be marketed as Tauvid.
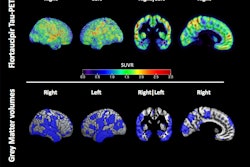
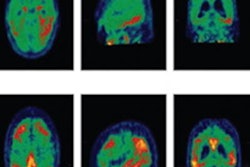
Tauvid is indicated for adult patients with cognitive impairment who are being evaluated for Alzheimer's disease. The PET radiopharmaceutical helps clinicians estimate the density and distribution of tau neurofibrillary tangles in the brain, which are a marker of Alzheimer's disease. The FDA said the approval was the first for a radiopharmaceutical to image tau pathology.
In announcing the approval on May 28, the FDA noted that while the agency has previously approved imaging agents for amyloid pathology -- another neuropathological hallmark of Alzheimer's disease -- the Tauvid approval is the first for imaging of tau.
"This approval will provide healthcare professionals with a new type of brain scan to use in patients being evaluated for Alzheimer's disease," said Dr. Charles Ganley, director of the FDA's Office of Specialty Medicine in the Center for Drug Evaluation and Research.
Tau and amyloid are both acknowledged as hallmarks of Alzheimer's disease. In Alzheimer's patients, tau proteins can develop inside neurons in the brain, creating neurofibrillary tangles. After injection, Tauvid binds to locations in the brain that are associated with the misfolding of tau proteins; these locations can then be identified on PET scans.
The FDA noted that two clinical studies evaluated the safety and efficacy of Tauvid, with five readers in each study interpreting images. In one study, 156 terminally ill patients were enrolled to receive Tauvid imaging; they also agreed to donate their brains postmortem.
In 64 patients who died within nine months of getting Tauvid scans, pathologists evaluated neurofibrillary tangles in the postmortem brain tissue and the results were compared with readers interpreting Tauvid images. Researchers found that the readers had a high probability of correctly evaluating patients with tau pathology and an average-to-high probability of correctly assessing patients without tau pathology.
In the second study, the same patients as the original study were evaluated, along with an additional 18 patients with terminal illness. Also, 159 patients with cognitive impairment who were being evaluated for Alzheimer's disease were included as representative of the target population for Tauvid. The goal of the study was to assess how well the interpretations by the Tauvid readers agreed with each other.
The researchers found agreement of 0.87 across all 241 patients, with 1 representing perfect agreement and 0 representing no agreement. In a subanalysis of the population, researchers had an agreement of 0.82 for a subgroup of 82 terminally ill patients who were diagnosed after death and 0.90 for patients in the target population.
The FDA approved Tauvid using its priority review process, in which the agency's goal is to act on an application within six months of when the agency determines that the drug, once approved, would significantly improve the safety or effectiveness of treating, diagnosing, or preventing a serious disease.