Imaging specialists who visually interpreted flortaucipir-PET images were able to determine a person's risk of progressing to Alzheimer's and mild cognitive impairment within the next 18 months, according to a study published February 15 in JAMA Neurology.
Researchers from several academic institutions and Avid Radiopharmaceuticals, which developed and markets flortaucipir, found that approximately 70% of study participants with characteristics of advanced Alzheimer's disease on visual interpretation of baseline PET scans experienced "clinically meaningful deterioration" 18 months later. By comparison, less than half of the subjects with nonadvanced Alzheimer's traits had similar levels of cognitive deterioration.
"[D]ata from two independent studies consistently demonstrated that participants with a flortaucipir advanced Alzheimer's disease tau pattern are at a higher risk of clinically meaningful deterioration," wrote the authors led by Dr. Ming Lu, director of statistics at Avid Radiopharmaceuticals. "Thus, flortaucipir-PET, interpreted with a clinically applicable visual method, could provide helpful information regarding anticipated clinical decline in the management of patients being assessed for causes of cognitive impairment."
The PET tracer flortaucipir is designed to detect the accumulation of tau tangles in brain regions associated with Alzheimer's and dementia. Avid Radiopharmaceuticals markets the tracer under the brand name Tauvid and received FDA clearance in May 2020 for its use to image tau pathology.
While previous research studies have detailed how flortaucipir can uncover this precursor to Alzheimer's, how well the use of the tracer with PET translates to the clinical setting is somewhat in question. The researchers also noted that previous studies have relied on quantitative measures of flortaucipir.
But the researchers posited that a recently developed visual interpretation method might find broader clinical use and make the radiopharmaceutical more available to clinicians. The method uses a color scale to represent counts of radiopharmaceutical uptake in the brain, and readers score brain regions as negative or positive based on an elevated flortaucipir signal of more than 65% above the cerebellar signal. The readers then are able to score whether brain regions have patterns of tracer uptake that either are or are not consistent with Alzheimer's disease.
To determine the value of this approach, the researchers looked at two prospective, open-label, longitudinal studies. One study covered 160 participants (50 years and older) from 26 U.S. sites from December 2014 to July 2017, while a second study included 205 subjects (ages 55 to 85) from 68 worldwide sites.
All of the participants underwent baseline flortaucipir-PET scans to determine tracer uptake and tau presence. Cognitive conditions were evaluated 18 months later for 363 subjects using a clinical dementia rating (CDR). The CDR is based on a five-point scale (0, 0.5, 1, 2, 3) to gauge cognitive performance, with 0 as normal and 3 as severe dementia.
"Both studies tested the same hypotheses: baseline tau status, determined by flortaucipir visual interpretation patterns, would predict the risk of clinical decline over 18 months of follow-up," the authors explained.
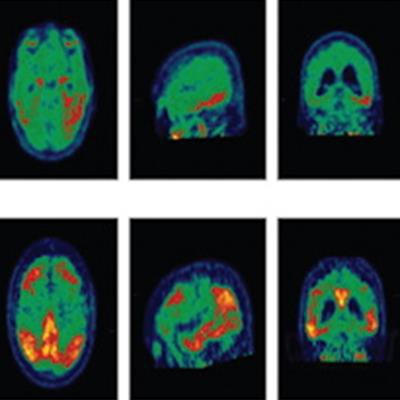
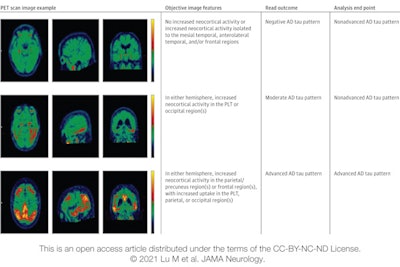 Image displays categories used in the visual interpretation of flortaucipir PET scans. Three cases represent the three levels of visual reads and corresponding analysis end-point calculation. Image courtesy of JAMA Neurology.
Image displays categories used in the visual interpretation of flortaucipir PET scans. Three cases represent the three levels of visual reads and corresponding analysis end-point calculation. Image courtesy of JAMA Neurology.Baseline flortaucipir-PET scans showed that 240 subjects (66%) had patterns associated with advanced Alzheimer's disease, which presumably would them more susceptible to the disease. At the 18-month follow-up, 210 subjects (87%) maintained their advanced Alzheimer's pattern, with 147 (70%) of them having an increase of 1 point or more in their CDR, indicating their dementia had worsened.
Among the 123 people with a nonadvanced Alzheimer's pattern, 105 subjects (85%) had the same nonadvanced characteristic 18 months later. Of that sample, only 48 participants (45%) saw their CDR increase by 1 point or more, meaning their conditions also had deteriorated.
Given the results, Lu and colleagues concluded that "an advanced Alzheimer's flortaucipir pattern was associated with increased risk of clinical progression over 18 months, as demonstrated by higher event rates and larger mean deteriorations across all the clinical end point measurements."
They added, however, that the findings "must be taken in context of other risk factors patients have for [dementia] progression and that we studied only 18 months of change in a disease that may have a 20- to 30-year natural history."
Disclosure: Lu and several study co-authors are employees of Eli Lilly and/or Avid Radiopharmaceuticals and minor shareholders of Eli Lilly.