PET/MRI with the F-18 fluciclovine radiotracer shows promise for initial staging of high-risk prostate cancer and for evaluating response to androgen deprivation therapy (ADT), according to a pilot study published online October 14 in the American Journal of Roentgenology.
The study findings suggest an accessible option for clinicians and their patients for managing prostate cancer, wrote a team led by Dr. Samuel Galgano of the University of Alabama at Birmingham.
"Given the [U.S. Food and Drug Administration] approval and widespread availability of F-18 fluciclovine, the findings could have impact in the immediate future in guiding initial management of patients with prostate cancer," the group noted.
Prostate cancer treatment has improved over the years, but rates of recurrence remain high due to small-volume metastatic disease missed on conventional imaging, according to the investigators. That's why it's important to find these lesions at initial diagnosis.
MRI alone is valuable for identifying prostate lesions, but it often misses smaller ones and/or nodal metastases. So Galgano's team explored the efficacy of combining PET with F-18 fluciclovine (Axumin, Blue Earth Diagnostics) with MRI for initial staging of high-risk prostate cancer and for assessing patients' response to ADT.
The study included 14 men with newly diagnosed, high-risk prostate cancer and negative or unclear conventional staging imaging. All 14 patients underwent F-18 fluciclovine PET/MRI imaging and multiparametric prostate MRI between January 2018 and February 2019; 12 had another PET/MRI exam after surgery or between ADT and radiotherapy.
Three radiologist readers rated identification of the primary lesion and nodal metastases using a 0 to 3 Likert scale and also two measures of standardized uptake values (SUV), including measured SUVmean and SUVmax, as well as volume of the MRI-identified lesion and SUVmax of suspicious lymph nodes on PET before and after ADT.
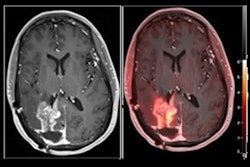
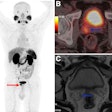
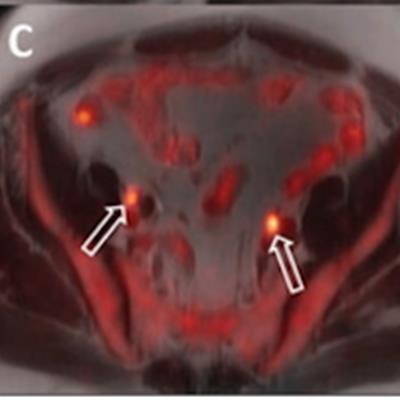
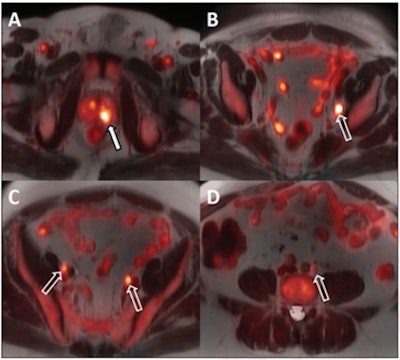 A 64-year-old man with biopsy-proven cancer rated as Gleason 4+5 in the left base and serum prostate-specific antigen of 11.1 ng/mL and equivocal conventional staging with 1.3-cm (borderline enlarged) left internal iliac lymph node. Fused F-18 fluciclovine PET/MR images demonstrate high focal uptake within the left prostate base (A, solid arrow) and left internal iliac lymph node (B, open arrow), as well as additional subcentimeter bilateral external iliac (C, open arrows) and left common iliac lymph nodes (D, open arrow) suspicious for metastatic disease. Images and caption courtesy of the American Journal of Roentgenology.
A 64-year-old man with biopsy-proven cancer rated as Gleason 4+5 in the left base and serum prostate-specific antigen of 11.1 ng/mL and equivocal conventional staging with 1.3-cm (borderline enlarged) left internal iliac lymph node. Fused F-18 fluciclovine PET/MR images demonstrate high focal uptake within the left prostate base (A, solid arrow) and left internal iliac lymph node (B, open arrow), as well as additional subcentimeter bilateral external iliac (C, open arrows) and left common iliac lymph nodes (D, open arrow) suspicious for metastatic disease. Images and caption courtesy of the American Journal of Roentgenology.Both F-18 fluciclovine PET/MRI and MRI alone accurately identified biopsy-proven prostate lesions in all 14 patients. MRI identified nodal metastases in three patients and F-18 fluciclovine PET/MRI identified metastases in seven patients.
| MRI vs. F-18 fluciclovine PET/MRI for initial prostate cancer staging | ||
| Finding | MRI | PET/MRI |
| Primary lesion | 14 (100%) | 14 (100%) |
| Seminal vesicle invasion | 14% | 29% |
| Pelvic nodal metastases | 21% | 50% |
| Extraregional metastases | Not applicable | 14% |
The study results could positively impact how prostate cancer patients are managed, according to the team.
"F-18 fluciclovine PET/MRI allows for a comprehensive initial staging of men with high-risk prostate cancer in a single session, and the components of the examination provide complementary information," the team concluded. "Given the FDA approval of F-18 fluciclovine, this study could have widespread impact in the immediate future [for] guiding initial management of patients with prostate cancer."