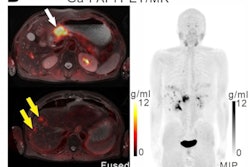
Sofie Biosciences recently received clearance from the U.S. Food and Drug Administration (FDA) to proceed with a clinical trial for a PET radiotracer based on fibroblast activation protein inhibitor (FAPI).
The company will now lead a phase II, multicenter, single-blind, nonrandomized study of its gallium-68 FAPI-46 PET radiotracer for imaging patients with pancreatic ductal adenocarcinoma. This comes after a 30-day review with the FDA.