PET imaging can help predict whether patients will respond to chemotherapy for pancreatic cancer before surgery, according to a study published April 8 in Clinical Radiology.
Japanese researchers analyzed imaging data from a group of patients with pancreatic adenocarcinoma who underwent F-18 FDG-PET/CT scans. They found PET radiotracer uptake values identified which patients were more likely to respond to initial chemotherapy prior to surgery.
"F-18 FDG-PET may be useful as a biomarker to predict the pathological response to [neoadjuvant chemotherapy] in patients with [pancreatic adenocarcinoma]," wrote corresponding author Dr. Akihiro Nishie, an associate professor of clinical radiology at Kyushu University in Fukuoka.
Pancreatic cancer has a poor prognosis. In 2018, pancreatic cancer was the seventh most prevalent type of cancer, with a mortality of 4.4 deaths per 100,000 people. Patients often receive chemotherapy as a first step to shrink tumors (neoadjuvant chemotherapy) and then continue treatment after tumors are removed.
Predicting whether patients respond to neoadjuvant chemotherapy based on F-18 FDG-PET imaging could have major implications for clinicians when deciding on subsequent treatment approaches, according to the authors.
In this retrospective study, Nishie and colleagues identified patients with pancreatic cancer who underwent F-18 FDG-PET/CT scans between April 2018 and August 2020 at their hospital. They included 28 patients who had surgery to remove tumors after initial neoadjuvant chemotherapy. Patients were split into groups of responders and nonresponders based on laboratory biopsy findings.
Next, the researchers measured maximum standardized uptake values (SUVmax) of the tumors on F-18 FDG-PET and compared the values among responders and nonresponders. The diagnostic performance of SUVmax in distinguishing between the two groups was evaluated using receiver operating characteristic (ROC) analysis.
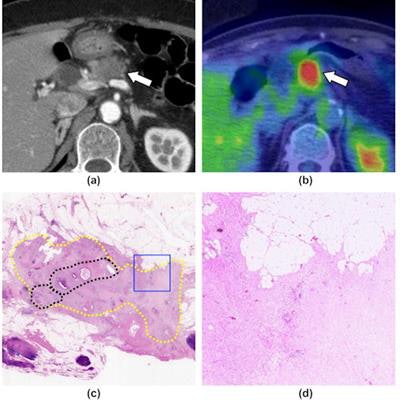
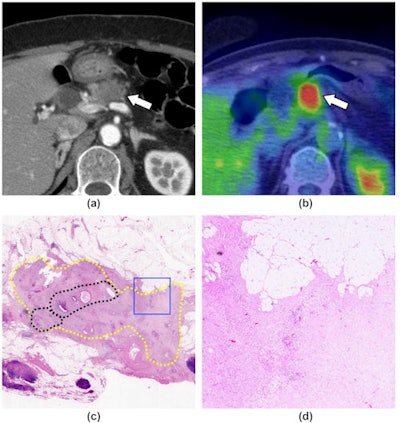 A 71-year-old woman with pancreatic ductal adenocarcinoma, shown as a representative case of the response group. (a) A contrast-enhanced CT image (pancreatic parenchymal phase) shows a hypoattenuating mass at the body of the pancreas (arrow). (b) An FDG-PET/CT image shows FDG uptake in the mass (arrow, SUVmax = 9.43). (c) A loupe view using hematoxylin and eosin staining shows the cancer remnant area (yellow dotted circles). A relatively dense area of residual cancer cells is observed in the center of the tumor (black dotted circles). Most of the periphery of the tumor is covered with fibrosis. (d) A low magnification view (×40) using hematoxylin and eosin staining, which corresponds to the blue square in (c), shows the presence of a few cancer cells and extensive fibrosis (grade 2). Image courtesy of Clinical Radiology.
A 71-year-old woman with pancreatic ductal adenocarcinoma, shown as a representative case of the response group. (a) A contrast-enhanced CT image (pancreatic parenchymal phase) shows a hypoattenuating mass at the body of the pancreas (arrow). (b) An FDG-PET/CT image shows FDG uptake in the mass (arrow, SUVmax = 9.43). (c) A loupe view using hematoxylin and eosin staining shows the cancer remnant area (yellow dotted circles). A relatively dense area of residual cancer cells is observed in the center of the tumor (black dotted circles). Most of the periphery of the tumor is covered with fibrosis. (d) A low magnification view (×40) using hematoxylin and eosin staining, which corresponds to the blue square in (c), shows the presence of a few cancer cells and extensive fibrosis (grade 2). Image courtesy of Clinical Radiology.Image analysis was performed by one radiologist who had 25 years of experience in nuclear medicine.
The nonresponse group consisted of 23 cases, while the response group included five cases, according to the findings. The researchers found that the mean SUVmax of the response group was higher than that of the nonresponse group (9.00 vs. 4.26; p < 0.001). The optimal cutoff value of SUVmax was 9.28 for distinguishing between the two groups.
In addition, ROC analysis revealed that F-18 FDG-PET/CT SUVmax has a sensitivity of 80%, a specificity of 95.7%, and accuracy of 92.9% when predicting patients in the response group.
"This study showed that the mean SUVmax of [pancreatic adenocarcinomas] that responded to [neoadjuvant chemotherapy] was significantly higher than that of [pancreatic adenocarcinomas] that did not respond well," the authors stated.
In recent years, neoadjuvant chemotherapy has become an essential treatment for resectable pancreatic cancer, but the factors predicting responses to neoadjuvant chemotherapy are unclear, the researchers wrote.
Despite the small numbers of patients included, this study is clinically significant because it establishes that patients with pancreatic cancer and high SUVmax on F-18 FDG PET/CT scans respond well to neoadjuvant chemotherapy, the team wrote.
"Baseline [pancreatic adenocarcinoma] SUVmax might help guide personalized treatment decisions in the future," Nishie and colleagues concluded.