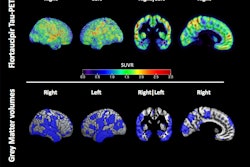
PET radiotracer uptake in women's skulls may cloud imaging results showing signs of early Alzheimer's disease, researchers found in a study published August 11 in the Journal of Nuclear Medicine.
A team at Washington University in St. Louis, MO, singled out skull tau uptake (a suspected "off-target" phenomenon) from PET images in normal and mildly cognitively impaired men and women participating in Alzheimer's disease trials. The group found high skull uptake likely due to low bone density erroneously contributed to results in 16% of participants, mostly women.
"As signal in skull bone can impact quantitative analyses and differs across sex, it should be explicitly addressed in studies of aging and [Alzheimer's disease]," wrote corresponding author Brian Gordon, PhD, an assistant professor of radiology at the university's Alzheimer Disease Research Center.
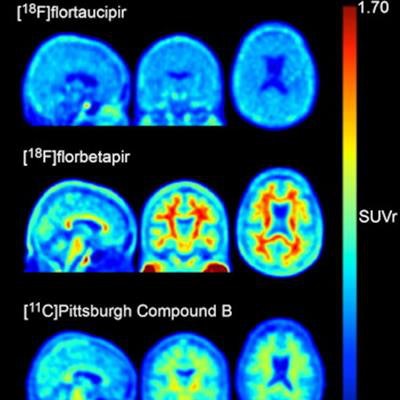
Current guidelines for determining whether a living person has Alzheimer's disease and, if so, the severity of the condition, are based largely on PET imaging of accumulated amounts of beta-amyloid plaque and neurofibrillary tau protein tangles in the brain. PET radiotracers are designed to bind to amyloid plaque and tau protein, and thus signal affected brain areas.
Off-target binding in PET studies refers to the phenomenon in which radiotracers bind to targets (proteins or other molecules in the body) other than those for which they were meant. Previous studies have identified notable off-target flortaucipir F-18 binding in the choroid plexus, basal ganglia, and brainstem, none of which are believed to be related to Alzheimer's disease pathology.
Another off-target region seldom discussed, but nonetheless identified, is skull bone, according to the authors. In this study, the researchers hypothesized that high tau skull binding could adversely impact quantification of Alzheimer's disease in early stages.
The group first created "skull masks" from CT scans to define regions of interest (ROIs) in 313 cognitively normal and mildly impaired men (n = 196) and women (n = 177). All subjects underwent amyloid PET (F-18 florbetapir and Pittsburgh compound B) and tau PET imaging as part of their participation in trials.
The masks were then used to quantify skull uptake among subjects of flortaucipir F-18 (Tauvid, Avid Radiopharmaceuticals), which is the most widely used tau PET tracer.
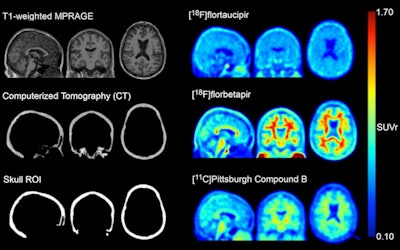 MR, CT, and standardized uptake value ratio (SUVr) images from an amyloid-negative participant with high radiotracer skull binding. Images are in MNI-152 space. Image courtesy of the Journal of Nuclear Medicine.
MR, CT, and standardized uptake value ratio (SUVr) images from an amyloid-negative participant with high radiotracer skull binding. Images are in MNI-152 space. Image courtesy of the Journal of Nuclear Medicine.An analysis revealed that 50 out of 313 (16%) flortaucipir F-18 scans had high levels of skull signal. The majority were female (n = 41, 82%) and in women, lower skull density was related to higher flortaucipir F-18 skull signal. The skull signal did not substantially correlate with other known off-target regions and was consistent over longitudinal scans, according to the results.
Importantly, in beta-amyloid negative, but not positive, individuals, flortaucipir F-18 skull binding impacted quantitative estimates in temporal brain regions, the researchers noted.
"Skull signal was observed primarily in younger women with decreased skull bone density, was a stable feature across time and tracers, and erroneously elevated quantitative measures of early tau accumulation in [Alzheimer's disease]," they wrote.
The authors noted limitations of the study, namely that comprehensive participant medical histories were not available, and thus an investigation into the role, if any, of medications or patient medical conditions was not possible.
"Future work should extend the presented analyses to other cohorts to elucidate the frequency of [flortaucipir F-18] skull binding across multiple longitudinal studies and the biological factors that influence its presentation," Gordon and colleagues concluded.